
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

Dopamine is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones. These pathways and cell groups form a dopamine system which is neuromodulatory.
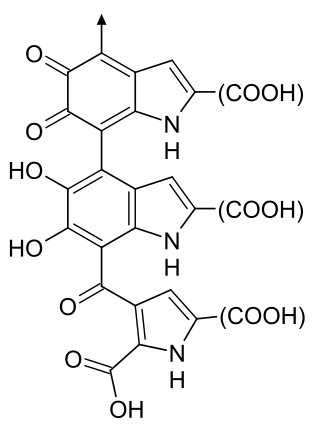
Melanin consist of oligomers or polymers arranged in a disordered manner which among other functions provide the pigments of many organisms. Melanin pigments are produced in a specialized group of cells known as melanocytes. They have been described as "among the last remaining biological frontiers with the unknown".

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

The locus coeruleus (LC), also spelled locus caeruleus or locus ceruleus, is a nucleus in the pons of the brainstem involved with physiological responses to stress and panic. It is a part of the reticular activating system.
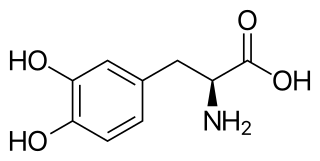
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.
Neuromodulation is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons. Neuromodulators typically bind to metabotropic, G-protein coupled receptors (GPCRs) to initiate a second messenger signaling cascade that induces a broad, long-lasting signal. This modulation can last for hundreds of milliseconds to several minutes. Some of the effects of neuromodulators include: altering intrinsic firing activity, increasing or decreasing voltage-dependent currents, altering synaptic efficacy, increasing bursting activity and reconfigurating synaptic connectivity.
Action selection is a way of characterizing the most basic problem of intelligent systems: what to do next. In artificial intelligence and computational cognitive science, "the action selection problem" is typically associated with intelligent agents and animats—artificial systems that exhibit complex behaviour in an agent environment. The term is also sometimes used in ethology or animal behavior.
The pars compacta (SNpc) is one of two subdivisions of the substantia nigra of the midbrain ; it is situated medial to the pars reticulata. It is formed by dopaminergic neurons. It projects to the striatum and portions of the cerebral cortex. It is functionally involved in fine motor control.
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a neurotoxic synthetic organic compound used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain.
Catecholaminergic cell groups refers to collections of neurons in the central nervous system that have been demonstrated by histochemical fluorescence to contain one of the neurotransmitters dopamine or norepinephrine. Thus, it represents the combination of dopaminergic cell groups and noradrenergic cell groups. Some authors include in this category 'putative' adrenergic cell groups, collections of neurons that stain for PNMT, the enzyme that converts norepinephrine to epinephrine (adrenaline).
Dopaminergic cell groups, DA cell groups, or dopaminergic nuclei are collections of neurons in the central nervous system that synthesize the neurotransmitter dopamine. In the 1960s, dopaminergic neurons or dopamine neurons were first identified and named by Annica Dahlström and Kjell Fuxe, who used histochemical fluorescence. The subsequent discovery of genes encoding enzymes that synthesize dopamine, and transporters that incorporate dopamine into synaptic vesicles or reclaim it after synaptic release, enabled scientists to identify dopaminergic neurons by labeling gene or protein expression that is specific to these neurons.
Gene therapy in Parkinson's disease consists of the creation of new cells that produce a specific neurotransmitter (dopamine), protect the neural system, or the modification of genes that are related to the disease. Then these cells are transplanted to a patient with the disease. There are different kinds of treatments that focus on reducing the symptoms of the disease but currently there is no cure.

The pathophysiology of Parkinson's disease is death of dopaminergic neurons as a result of changes in biological activity in the brain with respect to Parkinson's disease (PD). There are several proposed mechanisms for neuronal death in PD; however, not all of them are well understood. Five proposed major mechanisms for neuronal death in Parkinson's Disease include protein aggregation in Lewy bodies, disruption of autophagy, changes in cell metabolism or mitochondrial function, neuroinflammation, and blood–brain barrier (BBB) breakdown resulting in vascular leakiness.

Cell-based therapies for Parkinson's disease include various investigational procedures which transplant specific populations of cells into the brains of people with Parkinson's disease. The investigation of cell transplantation therapies followed the discovery that the death of dopaminergic neurons in the substantia nigra pars compacta resulted in the motor symptoms of the disease. Thus, cell transplantation has focused on various dopamine producing cells throughout the body.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.















