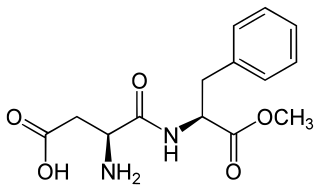
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with the trade names NutraSweet, Equal, and Canderel. First submitted for approval as a food ingredient in 1974, aspartame was approved by the US Food and Drug Administration (FDA) in 1981.

Amygdalin is a naturally occurring chemical compound found in many plants, most notably in the seeds (kernels) of apricots, bitter almonds, apples, peaches, cherries, and plums.

A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in order to increase the quantity of their consumption. The class of nutrient compounds includes vitamins, minerals, fiber, fatty acids, and amino acids. Dietary supplements can also contain substances that have not been confirmed as being essential to life, but are marketed as having a beneficial biological effect, such as plant pigments or polyphenols. Animals can also be a source of supplement ingredients, such as collagen from chickens or fish for example. These are also sold individually and in combination, and may be combined with nutrient ingredients. The European Commission has also established harmonized rules to help insure that food supplements are safe and appropriately labeled.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.
Xango, LLC, was a privately owned Lehi, Utah-based multi-level marketing company founded in 2002. It was acquired by Zija International in May 2017. The company marketed and distributed Xango juice, a blended juice product consisting of mangosteen and other juices, and skin care, personal care, energy supplement and nutritional supplement products. The company was warned in 2006 by the FDA for illegally marketing more than 20 human health benefits for Xango juice.

Morinda citrifolia is a fruit-bearing tree in the coffee family, Rubiaceae. Its native range extends across Southeast Asia and Australasia, and was spread across the Pacific by Polynesian sailors. The species is now cultivated throughout the tropics and widely naturalized. Among some 100 names for the fruit across different regions are the more common English names of great morinda, Indian mulberry, noni, beach mulberry, vomit fruit and cheese fruit.

Goji, goji berry, or wolfberry is the fruit of either Lycium barbarum or Lycium chinense, two closely related species of boxthorn in the nightshade family, Solanaceae. L. barbarum and L. chinense fruits are similar but can be distinguished by differences in taste and sugar content.
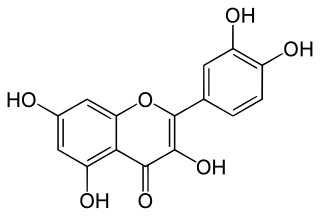
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

Cranberry juice is the liquid juice of the cranberry, often manufactured to contain sugar, water, and other fruit juices—especially when labeled as 'Cranberry Cocktail'. Cranberry—a fruit native to North America—is recognized for its bright red color, tart taste, and versatility for product manufacturing. Major cranberry products include cranberry juice, dried cranberry, cranberry sauce, frozen cranberry, cranberry powder, and dietary supplements containing cranberry extracts.
POM Wonderful, LLC is a private company which sells an eponymous brand of beverages and fruit extracts. It was founded in 2002 by the billionaire industrial agriculture couple Stewart and Lynda Rae Resnick. Through The Wonderful Company, their holding company, they are also affiliated with Teleflora, FIJI Water, pesticide manufacturer Suterra, and Paramount Agribusiness. In 2010, the company was warned by the FDA for making false health claims and for marketing statements that promoted their products as unauthorized drugs.

Morinda is a multi-level marketing company based in American Fork, Utah that sells Tahitian Noni juice and other products made from the noni plant. The company was founded in 1996 and has manufacturing facilities in Tahiti, Japan, China, Germany, and Utah. Morinda, formerly known as Tahitian Noni International and Morinda Bioactives, was a subsidiary of Morinda Holdings, Inc. prior to merging with and becoming a wholly owned subsidiary of New Age Beverages Corporation in December 2019.
Chromium deficiency is described as the consequence of an insufficient dietary intake of the mineral chromium. Chromium was first proposed as an essential element for normal glucose metabolism in 1959, and was widely accepted as being such by the 1990s. Cases of deficiency were described in people who received all of their nutrition intravenously for long periods of time.
Superfood is a marketing term for food claimed to confer health benefits resulting from an exceptional nutrient density. The term is not commonly used by experts, dietitians and nutrition scientists, most of whom dispute that particular foods have the health benefits claimed by their advocates. Even without scientific evidence of exceptional nutrient content, many new, exotic, and foreign fruits or ancient grains are marketed under the term – or superfruit or supergrain respectively – after being introduced or re-introduced to Western markets.

For the parent molecule 9,10-anthraquinone, see anthraquinone
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and hence is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.

Medical foods are foods that are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone. In the United States they were defined in the Food and Drug Administration's 1988 Orphan Drug Act Amendments and are subject to the general food and safety labeling requirements of the Federal Food, Drug, and Cosmetic Act. In Europe the European Food Safety Authority established definitions for "foods for special medical purposes" (FSMPs) in 2015.

MonaVie is a defunct, American multi-level marketing company that manufactured and distributed products made from blended fruit juice concentrates, powders, and purées. The company was the subject of several controversies. Health claims for its products had not been scientifically confirmed or approved by regulatory authorities, and its chairman had been previously involved in false health claims concerning another beverage company. According to Forbes, MonaVie's business plan resembled a pyramid scheme. In 2015, the company defaulted on a US$182 million loan and went into foreclosure. Florida-based Jeunesse Global took over MonaVie’s assets when it purchased the note for $15 million.

Added sugars or free sugars are sugar carbohydrates added to food and beverages at some point before their consumption. These include added carbohydrates, and more broadly, sugars naturally present in honey, syrup, and fruits. They can take multiple chemical forms, including sucrose, glucose (dextrose), and fructose.















