
Siboglinidae is a family of polychaete annelid worms whose members made up the former phyla Pogonophora and Vestimentifera. The family is composed of around 100 species of vermiform creatures which live in thin tubes buried in sediment (Pogonophora) or in tubes attached to hard substratum (Vestimentifera) at ocean depths ranging from 100 to 10,000 m. They can also be found in association with hydrothermal vents, methane seeps, sunken plant material, and whale carcasses.

Any worm that lives in a marine environment is considered a water worm. Marine worms are found in several different phyla, including the Platyhelminthes, Nematoda, Annelida, Chaetognatha, Hemichordata, and Phoronida. For a list of marine animals that have been called "sea worms", see sea worm.

Polychaeta is a paraphyletic class of generally marine annelid worms, commonly called bristle worms or polychaetes. Each body segment has a pair of fleshy protrusions called parapodia that bear many bristles, called chaetae, which are made of chitin. More than 10,000 species are described in this class. Common representatives include the lugworm and the sandworm or clam worm Alitta.

Riftia pachyptila, commonly known as the giant tube worm and less commonly known as the giant beardworm, is a marine invertebrate in the phylum Annelida related to tube worms commonly found in the intertidal and pelagic zones. R. pachyptila lives on the floor of the Pacific Ocean near hydrothermal vents. The vents provide a natural ambient temperature in their environment ranging from 2 to 30 °C, and this organism can tolerate extremely high hydrogen sulfide levels. These worms can reach a length of 3 m, and their tubular bodies have a diameter of 4 cm (1.6 in).

A whale fall occurs when the carcass of a whale has fallen onto the ocean floor, typically at a depth greater than 1,000 m (3,300 ft), putting them in the bathyal or abyssal zones. On the sea floor, these carcasses can create complex localized ecosystems that supply sustenance to deep-sea organisms for decades. In some circumstances, particularly in cases with lower water temperatures, they can be found at much shallower depths, with at least one natural instance recorded at 150 m and multiple experimental instances in the range of 30-382 m. Whale falls were first observed in the late 1970s with the development of deep-sea robotic exploration. Since then, several natural and experimental whale falls have been monitored through the use of observations from submersibles and remotely operated underwater vehicles (ROVs) in order to understand patterns of ecological succession on the deep seafloor.

The Monterey Bay Aquarium Research Institute (MBARI) is a private, non-profit oceanographic research center in Moss Landing, California. MBARI was founded in 1987 by David Packard, and is primarily funded by the David and Lucile Packard Foundation. Christopher Scholin serves as the institute's president and chief executive officer, managing a work force of approximately 220 scientists, engineers, and operations and administrative staff.
Osedax mucofloris is a species of bathypelagic Polychaetes that is reported to sustain itself on the bones of dead whales. Translated from the mixed Greek and Latin used in scientific names, "Osedax mucofloris" literally means "snot-flower bone-eater", though the less-accurate "bone-eating snot-flower worm" seems to be the form actually used. The species is found in North East Atlantic where it is abundant. Osedax mucofloris have special root tissues that they use to pierce into the whale bones found on the seafloor. Although the patterns and mechanisms of this piercing, known as boring, is poorly understood, there is evidence that Osedax mucofloris have acidic mucopolysaccharides in the mucus of their root tissues that aids in the mechanism of boring through bones.
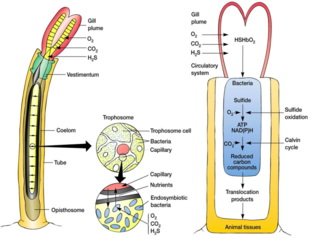
A trophosome is a highly vascularised organ found in some animals that houses symbiotic bacteria that provide food for their host. Trophosomes are contained by the coelom of tube worms and in the body of symbiotic flatworms of the genus Paracatenula.
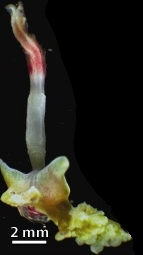
Osedax roseus is a species of bathypelagic polychaete worm that lives at abyssal depths and is able to sustain itself on the bones of dead whales. The species is found in the North East Pacific.
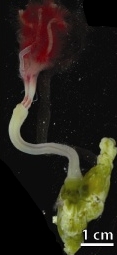
Osedax rubiplumus is a species of bathypelagic Polychaetes that is reported to sustain itself on the bones of dead whales.

Osedax frankpressi is a species of bathypelagic polychaete worm that lives on the seabed and sustains itself on the bones of dead whales. It can be found in the East North Pacific Ocean. The specific epithet is named in honor of Frank Press "for his distinguished service to science".
Osedax japonicus is a species of bathypelagic polychaete tube worm that lives at great depths on the seabed and is able to sustain itself on the bones of a dead whale. It was first described in 2006 from a sunken sperm whale carcase near Kyushu, Japan.

The annelids, also known as the segmented worms, are a large phylum, with over 22,000 extant species including ragworms, earthworms, and leeches. The species exist in and have adapted to various ecologies – some in marine environments as distinct as tidal zones and hydrothermal vents, others in fresh water, and yet others in moist terrestrial environments.
Osedax priapus is a species of bathypelagic annelid polychaete worms that consume the nutrients inside the bones of dead whales or other vertebrates.
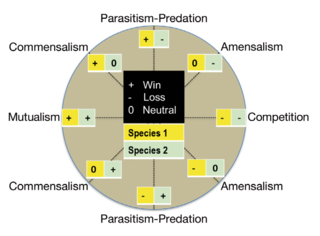
Microbial symbiosis in marine animals was not discovered until 1981. In the time following, symbiotic relationships between marine invertebrates and chemoautotrophic bacteria have been found in a variety of ecosystems, ranging from shallow coastal waters to deep-sea hydrothermal vents. Symbiosis is a way for marine organisms to find creative ways to survive in a very dynamic environment. They are different in relation to how dependent the organisms are on each other or how they are associated. It is also considered a selective force behind evolution in some scientific aspects. The symbiotic relationships of organisms has the ability to change behavior, morphology and metabolic pathways. With increased recognition and research, new terminology also arises, such as holobiont, which the relationship between a host and its symbionts as one grouping. Many scientists will look at the hologenome, which is the combined genetic information of the host and its symbionts. These terms are more commonly used to describe microbial symbionts.

Xenoturbella profunda, the purple sock or sock worm, is a marine, benthic, deep-water worm-like species that belongs to the genus Xenoturbella. It was discovered in eastern Pacific Ocean by a group of Californian and Australian scientists. The species was described in 2016 from seven specimens.
Kemp Caldera and Kemp Seamount form a submarine volcano south of the South Sandwich Islands, in a region where several seamounts are located. The seamount rises to a depth of 80 metres (260 ft) below sea level; the caldera has a diameter of 8.3 by 6.5 kilometres and reaches a depth of 1,600 metres (5,200 ft). The caldera contains several Hydrothermal vents, including white smokers and diffuse venting areas, which are host to chemolithotrophic ecological communities. The seamount and caldera, which were discovered by seafloor mapping in 2009, are part of the South Georgia and the South Sandwich Islands Marine Protected Area.

Lamellibrachia satsuma is a vestimentiferan tube worm that was discovered near a hydrothermal vent in Kagoshima Bay, Kagoshima at the depth of only 82 m (269 ft) the shallowest depth record for a vestimentiferan. Its symbiotic sulfur oxidizer bacteria have been characterised as ε-Proteobacteria and γ-Proteobacteria. Subspecies have been later found associated with cold seeps at Hatsushima in Sagami Bay and at the Daini Tenryu Knoll in the Nankai Trough with specimens obtained at up to 1,170 m (3,840 ft) depth.
Oligobrachia is a genus in the family Siboglinidae, commonly known as beard worms. These beard worms are typically found at spreading centers, hydrothermal vents, and undersea volcanoes. The siboglinidae are annelids which can be found buried in sediments. Beard worms do not necessarily exist at one specific part of the world's oceans, however, they are spread out all over the ocean floors as long as the surrounding environment is similar; these are known as metapopulations. Most commonly, these organisms are found at the bottom of the ocean floor, whether it be at a depth of roughly 25 meters or hundreds of meters. Oligobrachia can typically be found near hydrothermal vents and methane seeps. An important characteristic of this genus is that it lacks a mouth and gut. Therefore, it relies on symbiotic bacteria to provide the host organism with energy to survive. The majority of oligobrachia that have been observed have been found in the Arctic and other high-latitude areas of the world's oceans.
Frenulata, "beard worms", is a clade of Siboglinidae, "tube worms". They are one of four lineages with numerous species. They may be the most basal clade in the family. Despite being the first tube worms to be encountered and described, they remain the least studied group. This is because of their slender shape, they often get destroyed as a result of being caught as bycatch or poor preservation. They are found primarily in deep, muddy sediments, cold seeps, and anoxic firth sediments.
















