An allele, or allelomorph, is a variant of the sequence of nucleotides at a particular location, or locus, on a DNA molecule.

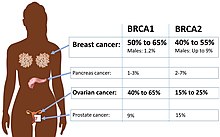
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosomal abnormality. Although polygenic disorders are the most common, the term is mostly used when discussing disorders with a single genetic cause, either in a gene or chromosome. The mutation responsible can occur spontaneously before embryonic development, or it can be inherited from two parents who are carriers of a faulty gene or from a parent with the disorder. When the genetic disorder is inherited from one or both parents, it is also classified as a hereditary disease. Some disorders are caused by a mutation on the X chromosome and have X-linked inheritance. Very few disorders are inherited on the Y chromosome or mitochondrial DNA.
The genotype of an organism is its complete set of genetic material. Genotype can also be used to refer to the alleles or variants an individual carries in a particular gene or genetic location. The number of alleles an individual can have in a specific gene depends on the number of copies of each chromosome found in that species, also referred to as ploidy. In diploid species like humans, two full sets of chromosomes are present, meaning each individual has two alleles for any given gene. If both alleles are the same, the genotype is referred to as homozygous. If the alleles are different, the genotype is referred to as heterozygous.

In genetics, the phenotype is the set of observable characteristics or traits of an organism. The term covers the organism's morphology, its developmental processes, its biochemical and physiological properties, its behavior, and the products of behavior. An organism's phenotype results from two basic factors: the expression of an organism's genetic code and the influence of environmental factors. Both factors may interact, further affecting the phenotype. When two or more clearly different phenotypes exist in the same population of a species, the species is called polymorphic. A well-documented example of polymorphism is Labrador Retriever coloring; while the coat color depends on many genes, it is clearly seen in the environment as yellow, black, and brown. Richard Dawkins in 1978 and then again in his 1982 book The Extended Phenotype suggested that one can regard bird nests and other built structures such as caddisfly larva cases and beaver dams as "extended phenotypes".

The genotype–phenotype distinction is drawn in genetics. The "Genotype" is an organism's full hereditary information. The "Phenotype" is an organism's actual observed properties, such as morphology, development, or behavior. This distinction is fundamental in the study of inheritance of traits and their evolution.

Dominance, in genetics, is defined as the interactions between alleles at the same locus on homologous chromosomes and the associated phenotype. In the case of complete dominance, one allele in a heterozygote individual completely overrides or masks the phenotypic contribution of the other allele. The overriding allele is referred to as dominant and the masked one recessive. Complete dominance, also referred to as Mendelian inheritance, follow Mendel’s laws of segregation. The first law states that each allele in a pair of genes is separated at random and have an equal probability of being transferred to the next generation, while the second law states that the distribution of allele variants is done independently of each other. However, this is not always the case as not all genes segregate independently and violations of this law are often referred to as “non-Mendelian inheritance”.
A quantitative trait locus (QTL) is a locus that correlates with variation of a quantitative trait in the phenotype of a population of organisms. QTLs are mapped by identifying which molecular markers correlate with an observed trait. This is often an early step in identifying the actual genes that cause the trait variation.
Genetic architecture is the underlying genetic basis of a phenotypic trait and its variational properties. Phenotypic variation for quantitative traits is, at the most basic level, the result of the segregation of alleles at quantitative trait loci (QTL). Environmental factors and other external influences can also play a role in phenotypic variation. Genetic architecture is a broad term that can be described for any given individual based on information regarding gene and allele number, the distribution of allelic and mutational effects, and patterns of pleiotropy, dominance, and epistasis.

Pleiotropy occurs when one gene influences two or more seemingly unrelated phenotypic traits. Such a gene that exhibits multiple phenotypic expression is called a pleiotropic gene. Mutation in a pleiotropic gene may have an effect on several traits simultaneously, due to the gene coding for a product used by a myriad of cells or different targets that have the same signaling function.
In genetics, expressivity is the degree to which a phenotype is expressed by individuals having a particular genotype. Alternatively, it may refer to the expression of a particular gene by individuals having a certain phenotype. Expressivity is related to the intensity of a given phenotype; it differs from penetrance, which refers to the proportion of individuals with a particular genotype that share the same phenotype.

Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type phenotype. Haploinsufficiency may arise from a de novo or inherited loss-of-function mutation in the variant allele, such that it yields little or no gene product. Although the other, standard allele still produces the standard amount of product, the total product is insufficient to produce the standard phenotype. This heterozygous genotype may result in a non- or sub-standard, deleterious, and (or) disease phenotype. Haploinsufficiency is the standard explanation for dominant deleterious alleles.

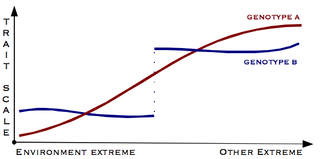
Gene–environment interaction is when two different genotypes respond to environmental variation in different ways. A norm of reaction is a graph that shows the relationship between genes and environmental factors when phenotypic differences are continuous. They can help illustrate GxE interactions. When the norm of reaction is not parallel, as shown in the figure below, there is a gene by environment interaction. This indicates that each genotype responds to environmental variation in a different way. Environmental variation can be physical, chemical, biological, behavior patterns or life events.
In genetics, concordance is the probability that a pair of individuals will both have a certain characteristic given that one of the pair has the characteristic. Concordance can be measured with concordance rates, reflecting the odds of one person having the trait if the other does. Important clinical examples include the chance of offspring having a certain disease if the mother has it, if the father has it, or if both parents have it. Concordance among siblings is similarly of interest: what are the odds of a subsequent offspring having the disease if an older child does? In research, concordance is often discussed in the context of both members of a pair of twins. Twins are concordant when both have or both lack a given trait. The ideal example of concordance is that of identical twins, because the genome is the same, an equivalence that helps in discovering causation via deconfounding, regarding genetic effects versus epigenetic and environmental effects.
Canalisation is a measure of the ability of a population to produce the same phenotype regardless of variability of its environment or genotype. It is a form of evolutionary robustness. The term was coined in 1942 by C. H. Waddington to capture the fact that "developmental reactions, as they occur in organisms submitted to natural selection...are adjusted so as to bring about one definite end-result regardless of minor variations in conditions during the course of the reaction". He used this word rather than robustness to consider that biological systems are not robust in quite the same way as, for example, engineered systems.

Transgenerational epigenetic inheritance is the transmission of epigenetic markers and modifications from one generation to multiple subsequent generations without altering the primary structure of DNA. Thus, the regulation of genes via epigenetic mechanisms can be heritable; the amount of transcripts and proteins produced can be altered by inherited epigenetic changes. In order for epigenetic marks to be heritable, however, they must occur in the gametes in animals, but since plants lack a definitive germline and can propagate, epigenetic marks in any tissue can be heritable.
Locus heterogeneity occurs when mutations at multiple genomic loci are capable of producing the same phenotype, and each individual mutation is sufficient to cause the specific phenotype independently. Locus heterogeneity should not be confused with allelic heterogeneity, in which a single phenotype can be produced by multiple mutations, all of which are at the same locus on a chromosome. Likewise, it should not be confused with phenotypic heterogeneity, in which different phenotypes arise among organisms with identical genotypes and environmental conditions. Locus heterogeneity and allelic heterogeneity are the two components of genetic heterogeneity.
Genocopy is a trait that is a phenotypic copy of a genetic trait but is caused by a different genotype. When a genetic mutation or genotype in one locus results in a phenotype similar to one that is known to be caused by another mutation or genotype in another locus, it is said to be a genocopy. However, genocopies may also be referred to as "genetic mimics", in which the same mutation or specific genotype can result in two unique phenotypes in two different patients. The term “Genocopy” was coined by Dr. H. Nachstheim in 1957, in which he discusses “false” phenocopies. In comparison to when a phenotype is the result of an environmental condition that had the same effect as a previously known genetic factor such as mutation. While offspring may inherit specific mutations or genotypes that result in genocopies, phenocopies are not heritable. Two types of elliptocytosis that are genocopies of each other, but are distinguished by the fact that one is linked to the Rh blood group locus and the other is not. The way to distinguish a recessive genocopy from a phenotype caused by a different allele would be by carrying out a test cross, breeding the two together, if they F1 hybrid segregates 1:2:1 then we can determine that it was a genocopy.
Oligogenic inheritance describes a trait that is influenced by a few genes. Oligogenic inheritance represents an intermediate between monogenic inheritance in which a trait is determined by a single causative gene, and polygenic inheritance, in which a trait is influenced by many genes and often environmental factors.

The genotype-first approach is a type of strategy used in genetic epidemiological studies to associate specific genotypes to apparent clinical phenotypes of a complex disease or trait. As opposed to “phenotype-first”, the traditional strategy that has been guiding genome-wide association studies (GWAS) so far, this approach characterizes individuals first by a statistically common genotype based on molecular tests prior to clinical phenotypic classification. This method of grouping leads to patient evaluations based on a shared genetic etiology for the observed phenotypes, regardless of their suspected diagnosis. Thus, this approach can prevent initial phenotypic bias and allow for identification of genes that pose a significant contribution to the disease etiology.
A human disease modifier gene is a modifier gene that alters expression of a human gene at another locus that in turn causes a genetic disease. Whereas medical genetics has tended to distinguish between monogenic traits, governed by simple, Mendelian inheritance, and quantitative traits, with cumulative, multifactorial causes, increasing evidence suggests that human diseases exist on a continuous spectrum between the two.