
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered, even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source.

High-temperature superconductors are defined as materials with critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at even colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first break through of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.
Palladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At room temperature, palladium hydrides may contain two crystalline phases, α and β. Pure α-phase exists at x < 0.017 whereas pure β-phase is realised for x > 0.58; intermediate x values correspond to α-β mixtures.
A room-temperature superconductor is a hypothetical material capable of displaying superconductivity at temperatures above 0 °C, which are commonly encountered in everyday settings. As of 2023, the material with the highest accepted superconducting temperature was highly pressurized lanthanum decahydride, whose transition temperature is approximately 250 K (−23 °C) at 200 GPa.
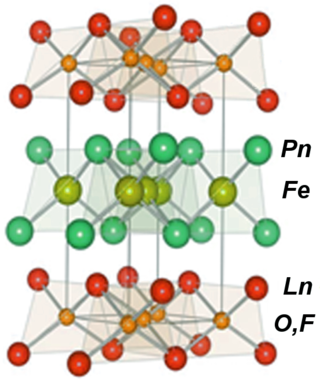
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..

Covalent superconductors are superconducting materials where the atoms are linked by covalent bonds. The first such material was boron-doped synthetic diamond grown by the high-pressure high-temperature (HPHT) method. The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
Iron(II) selenide refers to a number of inorganic compounds of ferrous iron and selenide (Se2−). The phase diagram of the system Fe–Se reveals the existence of several non-stoichiometric phases between ~49 at. % Se and ~53 at. % Fe, and temperatures up to ~450 °C. The low temperature stable phases are the tetragonal PbO-structure (P4/nmm) β-Fe1−xSe and α-Fe7Se8. The high temperature phase is the hexagonal, NiAs structure (P63/mmc) δ-Fe1−xSe. Iron(II) selenide occurs naturally as the NiAs-structure mineral achavalite.
CeCoIn5 ("Cerium-Cobalt-Indium 5") is a heavy-fermion superconductor with a layered crystal structure, with somewhat two-dimensional electronic transport properties. The critical temperature of 2.3 K is the highest among all of the Ce-based heavy-fermion superconductors.

Artem R. Oganov is a Russian theoretical crystallographer, mineralogist, chemist, physicist, and materials scientist. He is known mostly for his works on computational materials discovery and crystal structure prediction, studies of matter at extreme conditions, including matter of planetary interiors.
Yttrium hydride is a compound of hydrogen and yttrium. It is considered to be a part of the class of rare-earth metal hydrides. It exists in several forms, the most common being a metallic compound with formula YH2. YH2 has a face-centred cubic structure, and is a metallic compound. Under great pressure, extra hydrogen can combine to yield an insulator with a hexagonal structure, with a formula close to YH3. Hexagonal YH3 has a band gap of 2.6 eV. Under pressure of 12 GPa YH3 transforms to an intermediate state, and when the pressure increases to 22 GPa another metallic face-centred cubic phase is formed.

Mikhail Ivanovich Eremets is an experimentalist in high pressure physics, chemistry and materials science. He is particularly known for his research on superconductivity, having discovered the highest critical temperature of 250 K (-23 °C) for superconductivity in lanthanum hydride under high pressures. Part of his research contains exotic manifestations of materials such as conductive hydrogen, polymeric nitrogen and transparent sodium.

Disodium helide (Na2He) is a compound of helium and sodium that is stable at high pressures above 113 gigapascals (1,130,000 bar). It was first predicted using the USPEX crystal structure prediction algorithm and then synthesised in 2016.
Lanthanum decahydride is a polyhydride or superhydride compound of lanthanum and hydrogen (LaH10) that has shown evidence of being a high-temperature superconductor. It has a superconducting transition temperature TC around 250 K (−23 °C; −10 °F) at a pressure of 150 gigapascals (22×10^6 psi), and its synthesis required pressures above approximately 160 gigapascals (23×10^6 psi).
In chemistry, a hydridonitride is a chemical compound that contains hydride and nitride ions in a single phase. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and contain a larger proportion of metals.
An arsenide hydride or hydride arsenide is a chemical compound containing hydride (H−) and arsenide (As3−) ions in a single phase. They are in the class of mixed anion compounds.
Metallization pressure is the pressure required for a non-metallic chemical element to become a metal. Every material is predicted to turn into a metal if the pressure is high enough, and temperature low enough. Some of these pressures are beyond the reach of diamond anvil cells, and are thus theoretical predictions. Neon has the highest metallization pressure for any element.
Carbonaceous sulfur hydride (CSH) is a purported room-temperature superconductor that was announced in October 2020. The material is claimed to have a maximal superconducting transition temperature of 15 °C (59 °F) at a pressure of 267 gigapascals (GPa), though the validity of the claim has faced criticism. In September 2022 the article was retracted by Nature due to a non standard, user-defined data analysis calling into question the scientific validity of the claim. In July 2023 a second paper by the author was retracted from Physical Review Letters due to suspected data fabrication.
Ranga P. Dias is a researcher and academic who specializes in condensed matter physics. He is an assistant professor in Mechanical Engineering and Physics and Astronomy at the University of Rochester and a scientist at the Laboratory for Laser Energetics.







