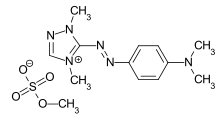
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.
In chemistry, a zwitterion, also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Polyene antimycotics, sometimes referred to as polyene antibiotics, are a class of antimicrobial polyene compounds that target fungi. These polyene antimycotics are typically obtained from some species of Streptomyces bacteria. Previously, polyenes were thought to bind to ergosterol in the fungal cell membrane and thus weakening it and causing leakage of K+ and Na+ ions, which could contribute to fungal cell death. However, more detailed studies of polyene molecular properties have challenged this model suggesting that polyenes instead bind and extract ergosterol directly from the cellular membrane thus disrupting the many cellular functions ergosterols perform. Amphotericin B, nystatin, and natamycin are examples of polyene antimycotics. They are a subgroup of macrolides.
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV.

Tetraphenylmethane is an organic compound consisting of a methane core with four phenyl substituents. It was first synthesized by Moses Gomberg in 1898.
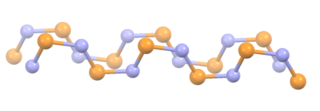
Polythiazyl, (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered and was also found to be a superconductor at very low temperatures. It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.
Nitrile hydratases are mononuclear iron or non-corrinoid cobalt enzymes that catalyse the hydration of diverse nitriles to their corresponding amides

Hexazine is an allotrope of nitrogen composed of 6 nitrogen atoms arranged in a ring-like structure analogous to that of benzene. As a neutral-charged species, it would be the final member of the azabenzene (azine) series, in which all of the methine groups of the benzene molecule have been replaced with nitrogen atoms. The two last members of this series, hexazine and pentazine, have not been observed, although all other members of the azine series have.
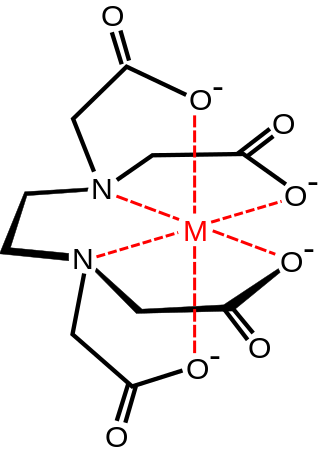
An aminopolycarboxylic acid is a chemical compound containing one or more nitrogen atoms connected through carbon atoms to two or more carboxyl groups. Aminopolycarboxylates that have lost acidic protons form strong complexes with metal ions. This property makes aminopolycarboxylic acids useful complexone in a wide variety of chemical, medical, and environmental applications.

Hans Kuhn was a Swiss chemist. He was professor emeritus for physical chemistry and former scientific director at the Max Planck Institute for Biophysical Chemistry in Göttingen.
These drugs are known in the UK as controlled drugs, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs are controlled by other laws.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.
Lysine carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction:
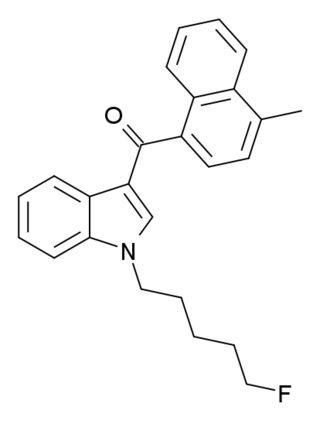
To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general structure as opposed to their specific identity. In this way new analogs are already controlled before they are even created. A large number of cannabinoids have been grouped into classes based on similarities in their chemical structure, and these classes have been widely adopted across a variety of jurisdictions.