
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes inside the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from computed tomography (CT) and positron emission tomography (PET) scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy.
Blood-oxygen-level-dependent imaging, or BOLD-contrast imaging, is a method used in functional magnetic resonance imaging (fMRI) to observe different areas of the brain or other organs, which are found to be active at any given time.
Magnetic resonance elastography (MRE) is a form of elastography that specifically leverages MRI to quantify and subsequently map the mechanical properties of soft tissue. First developed and described at Mayo Clinic by Muthupillai et al. in 1995, MRE has emerged as a powerful, non-invasive diagnostic tool, namely as an alternative to biopsy and serum tests for staging liver fibrosis.
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.
Kenneth Kin Man Kwong is a Hong Kong-born American nuclear physicist. He is a pioneer in human brain imaging. He received his bachelor's degree in Political Science in 1972 from the University of California, Berkeley. He went on to receive his Ph.D. in physics from the University of California, Riverside studying photon-photon collision interactions.
In vivo magnetic resonance spectroscopy (MRS) is a specialized technique associated with magnetic resonance imaging (MRI).
Signal enhancement by extravascular water protons, or SEEP, is a contrast mechanism for functional magnetic resonance imaging (fMRI), which is an alternative to the more commonly employed BOLD contrast. This mechanism for image contrast changes corresponding to changes in neuronal activity was first proposed by Dr. Patrick Stroman in 2001. SEEP contrast is based on changes in tissue water content which arise from the increased production of extracellular fluid and swelling of neurons and glial cells at sites of neuronal activity. Because the dominant sources of MRI signal in biological tissues are water and lipids, an increase in tissue water content is reflected by a local increase in MR signal intensity. A correspondence between BOLD and SEEP signal changes, and sites of activity, has been observed in the brain and appears to arise from the common dependence on changes in local blood flow to cause a change in blood oxygenation or to produce extracellular fluid. The advantage of SEEP contrast is that it can be detected with MR imaging methods which are relatively insensitive to magnetic susceptibility differences between air, tissues, blood, and bone. Such susceptibility differences can give rise to spatial image distortions and areas of low signal, and magnetic susceptibility changes in blood give rise to the BOLD contrast for fMRI. The primary application of SEEP to date has been fMRI of the spinal cord because the bone/tissue interfaces around the spinal cord cause poor image quality with conventional fMRI methods. The disadvantages of SEEP compared to BOLD contrast are that it reveals more localized areas of activity, and in the brain the signal intensity changes are typically lower, and it can therefore be more difficult to detect.
Functional magnetic resonance imaging (fMRI) of the spinal cord is an adaptation of the fMRI method that has been developed for use in the brain. Although the basic principles underlying the methods are the same, spinal fMRI requires a number of specific adaptations to accommodate the periodic motion of the spinal cord, the small cross-sectional dimensions and length of the spinal cord, and the fact that the magnetic field that is used for MRI varies with position in the spinal cord because of magnetic susceptibility differences between bone and tissues. Spinal fMRI has been used to produce maps of neuronal activity at most levels of the spinal cord in response to various stimuli, such as touch, vibration, and thermal changes, and with motor tasks. Research applications of spinal fMRI to date include studies of normal sensory and motor function, and studies of the effects of trauma and multiple sclerosis on the spinal cord.
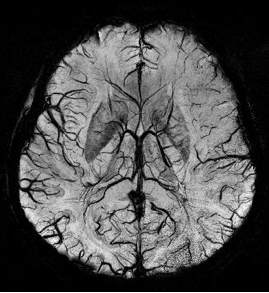
Susceptibility weighted imaging (SWI), originally called BOLD venographic imaging, is an MRI sequence that is exquisitely sensitive to venous blood, hemorrhage and iron storage. SWI uses a fully flow compensated, long echo, gradient recalled echo (GRE) pulse sequence to acquire images. This method exploits the susceptibility differences between tissues and uses the phase image to detect these differences. The magnitude and phase data are combined to produce an enhanced contrast magnitude image. The imaging of venous blood with SWI is a blood-oxygen-level dependent (BOLD) technique which is why it was referred to as BOLD venography. Due to its sensitivity to venous blood SWI is commonly used in traumatic brain injuries (TBI) and for high resolution brain venographies but has many other clinical applications. SWI is offered as a clinical package by Philips and Siemens but can be run on any manufacturer's machine at field strengths of 1.0 T, 1.5 T, 3.0 T and higher.

Magnetic resonance imaging (MRI) is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.

Real-time magnetic resonance imaging (RT-MRI) refers to the continuous monitoring ("filming") of moving objects in real time. Because MRI is based on time-consuming scanning of k-space, real-time MRI was possible only with low image quality or low temporal resolution. Using an iterative reconstruction algorithm these limitations have recently been removed: a new method for real-time MRI achieves a temporal resolution of 20 to 30 milliseconds for images with an in-plane resolution of 1.5 to 2.0 mm. Real-time MRI promises to add important information about diseases of the joints and the heart. In many cases MRI examinations may become easier and more comfortable for patients.

Magnetic resonance imaging of the brain uses magnetic resonance imaging (MRI) to produce high quality two-dimensional or three-dimensional images of the brain and brainstem as well as the cerebellum without the use of ionizing radiation (X-rays) or radioactive tracers.
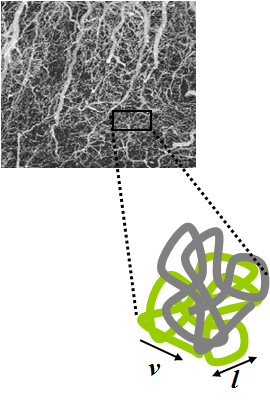
Intravoxel incoherent motion (IVIM) imaging is a concept and a method initially introduced and developed by Le Bihan et al. to quantitatively assess all the microscopic translational motions that could contribute to the signal acquired with diffusion MRI. In this model, biological tissue contains two distinct environments: molecular diffusion of water in the tissue, and microcirculation of blood in the capillary network (perfusion). The concept introduced by D. Le Bihan is that water flowing in capillaries mimics a random walk (Fig.1), as long as the assumption that all directions are represented in the capillaries is satisfied.
Synthetic MRI is a simulation method in Magnetic Resonance Imaging (MRI), for generating contrast weighted images based on measurement of tissue properties. The synthetic (simulated) images are generated after an MR study, from parametric maps of tissue properties. It is thereby possible to generate several contrast weightings from the same acquisition. This is different from conventional MRI, where the signal acquired from the tissue is used to generate an image directly, often generating only one contrast weighting per acquisition. The synthetic images are similar in appearance to those normally acquired with an MRI scanner.
The history of magnetic resonance imaging (MRI) includes the work of many researchers who contributed to the discovery of nuclear magnetic resonance (NMR) and described the underlying physics of magnetic resonance imaging, starting early in the twentieth century. One researcher was American physicist Isidor Isaac Rabi who won the Nobel Prize in Physics in 1944 for his discovery of nuclear magnetic resonance, which is used in magnetic resonance imaging. MR imaging was invented by Paul C. Lauterbur who developed a mechanism to encode spatial information into an NMR signal using magnetic field gradients in September 1971; he published the theory behind it in March 1973.

An MRI pulse sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
An MRI artifact is a visual artifact in magnetic resonance imaging (MRI). It is a feature appearing in an image that is not present in the original object. Many different artifacts can occur during MRI, some affecting the diagnostic quality, while others may be confused with pathology. Artifacts can be classified as patient-related, signal processing-dependent and hardware (machine)-related.
Amplified magnetic resonance imaging (aMRI) is an MRI method that is coupled with video magnification processing methods to amplify the subtle spatial variations in MRI scans and to enable better visualization of tissue motion. aMRI can enable better visualization of tissue motion to aid the in vivo assessment of the biomechanical response in pathology. It is thought to have potential for helping with diagnosing and monitoring a range of clinical implications in the brain and other organs, including in Chiari Malformation, brain injury, hydrocephalus, other conditions associated with abnormal intracranial pressure, cerebrovascular, and neurodegenerative disease.

Rolf Gruetter is a Swiss physicist and neurobiologist specialized in magnetic resonance, biomedical imaging and brain metabolism. He is a professor of physics at EPFL and the head of the Laboratory Functional and Metabolic Imaging at the School of Basic Sciences.
Magnetic resonance fingerprinting (MRF) is methodology in quantitative magnetic resonance imaging (MRI) characterized by a pseudo-randomized acquisition strategy. It involves creating unique signal patterns or 'fingerprints' for different materials or tissues after which a pattern recognition algorithm matches these fingerprints with a predefined dictionary of expected signal patterns. This process translates the data into quantitative maps, revealing information about the magnetic properties being investigated.


















