Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

Caspase recruitment domains, or caspase activation and recruitment domains (CARDs), are interaction motifs found in a wide array of proteins, typically those involved in processes relating to inflammation and apoptosis. These domains mediate the formation of larger protein complexes via direct interactions between individual CARDs. CARD domains are found on a strikingly wide range of proteins, including helicases, kinases, mitochondrial proteins, caspases, and other cytoplasmic factors.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.

Fas-associated protein with death domain (FADD), also called MORT1, is encoded by the FADD gene on the 11q13.3 region of chromosome 11 in humans.

G-protein-coupled receptor kinase 2 (GRK2) is an enzyme that in humans is encoded by the ADRBK1 gene. GRK2 was initially called Beta-adrenergic receptor kinase, and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK3(βARK2).

TNF receptor-associated factor 2 is a protein that in humans is encoded by the TRAF2 gene.
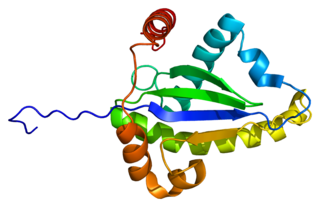
Tumor necrosis factor receptor type 1-associated DEATH domain protein is a protein that in humans is encoded by the TRADD gene.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

Protein kinase C beta type is an enzyme that in humans is encoded by the PRKCB gene.

Baculoviral IAP repeat-containing protein 2 is a protein that in humans is encoded by the BIRC2 gene.

Sequestosome-1 is a protein that in humans is encoded by the SQSTM1 gene. Also known as the ubiquitin-binding protein p62, it is an autophagosome cargo protein that targets other proteins that bind to it for selective autophagy. By interacting with GATA4 and targeting it for degradation, it can inhibit GATA-4 associated senescence and senescence-associated secretory phenotype.

Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) functions in a variety of cellular pathways related to both cell survival and death. In terms of cell death, RIPK1 plays a role in apoptosis and necroptosis. Some of the cell survival pathways RIPK1 participates in include NF-κB, Akt, and JNK.

Receptor-interacting serine/threonine-protein kinase 2 is an enzyme that in humans is encoded by the RIPK2 gene.

E3 ubiquitin-protein ligase RNF216 is an enzyme that in humans is encoded by the RNF216 gene.

Death domain-containing protein CRADD is a protein that in humans is encoded by the CRADD gene.

Receptor-interacting serine/threonine-protein kinase 3 is an enzyme that in humans is encoded by the RIPK3 gene.

Receptor-interacting serine/threonine-protein kinase 4 is an enzyme that in humans is encoded by the RIPK4 gene.

Dual serine/threonine and tyrosine protein kinase is an enzyme that in humans is encoded by the DSTYK gene.

Necroptosis is a programmed form of necrosis, or inflammatory cell death. Conventionally, necrosis is associated with unprogrammed cell death resulting from cellular damage or infiltration by pathogens, in contrast to orderly, programmed cell death via apoptosis. The discovery of necroptosis showed that cells can execute necrosis in a programmed fashion and that apoptosis is not always the preferred form of cell death. Furthermore, the immunogenic nature of necroptosis favors its participation in certain circumstances, such as aiding in defence against pathogens by the immune system. Necroptosis is well defined as a viral defense mechanism, allowing the cell to undergo "cellular suicide" in a caspase-independent fashion in the presence of viral caspase inhibitors to restrict virus replication. In addition to being a response to disease, necroptosis has also been characterized as a component of inflammatory diseases such as Crohn's disease, pancreatitis, and myocardial infarction.

Barettin is a brominated alkaloid made of a dehydrogenated brominated derivative of tryptophan linked by two peptide bonds to an arginine residue, forming a 2,5-diketopiperazine nucleus. It is a cyclic dipeptide.


















