Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.
Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.
The protein encoded by this gene is a member of the Ser/Thr protein kinase family. This protein is highly similar to the gene products of S. cerevisiae cdc28 and S. pombe cdc2. It is a catalytic subunit of the protein kinase complex that is important for cell cycle G1 phase progression. The activity of this kinase is restricted to the G1-S phase, which is controlled by the regulatory subunits D-type cyclins and CDK inhibitor p16INK4a. This kinase was shown to be responsible for the phosphorylation of retinoblastoma gene product (Rb). [4] Ser/Thr-kinase component of cyclin D-CDK4 (DC) complexes that phosphorylate and inhibit members of the retinoblastoma (RB) protein family including RB1 and regulate the cell-cycle during G1/S transition. Phosphorylation of RB1 allows dissociation of the transcription factor E2F from the RB/E2F complexes and the subsequent transcription of E2F target genes which are responsible for the progression through the G1 phase. Hypophosphorylates RB1 in early G1 phase. Cyclin D-CDK4 complexes are major integrators of various mitogenic and antimitogenic signals. Also phosphorylates SMAD3 in a cell-cycle-dependent manner and represses its transcriptional activity. Component of the ternary complex, cyclin D/CDK4/CDKN1B, required for nuclear translocation and activity of the cyclin D-CDK4 complex. [5]

Mutations in this gene as well as in its related proteins including D-type cyclins, p16(INK4a), CDKN2A and Rb were all found to be associated with tumorigenesis of a variety of cancers. One specific point mutation of CDK4 (R24C) was first identified in melanoma patients. This mutation was introduced also in animal models and its role as a cancer driver oncogene was studied thoroughly. Nowadays, deregulated CDK4 is considered to be a potential therapeutic target in some cancer types and various CDK4 inhibitors are being tested for cancer treatment in clinical trials. [6] [7]
Multiple polyadenylation sites of this gene have been reported. [4]
It is regulated by Cyclin D.
Ribociclib are US FDA approved CDK4 and CDK6 inhibitors for the treatment of estrogen receptor positive/ HER2 negative advanced breast cancer. [8]
See also CDK inhibitor for inhibitors of various CDKs.
Cyclin-dependent kinase 4 has been shown to interact with:


The cell cycle, or cell-division cycle, is the series of events that take place in a cell that causes it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.

Cyclin-dependent kinases (CDKs) are the families of protein kinases first discovered for their role in regulating the cell cycle. They are also involved in regulating transcription, mRNA processing, and the differentiation of nerve cells. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. In fact, yeast cells can proliferate normally when their CDK gene has been replaced with the homologous human gene. CDKs are relatively small proteins, with molecular weights ranging from 34 to 40 kDa, and contain little more than the kinase domain. By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase but its activity can be typically further modulated by phosphorylation and other binding proteins, like p27. CDKs phosphorylate their substrates on serines and threonines, so they are serine-threonine kinases. The consensus sequence for the phosphorylation site in the amino acid sequence of a CDK substrate is [S/T*]PX[K/R], where S/T* is the phosphorylated serine or threonine, P is proline, X is any amino acid, K is lysine, and R is arginine.
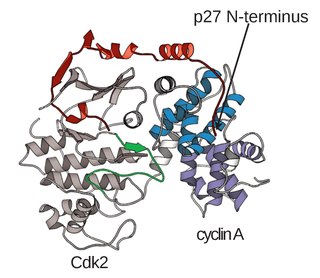
A cyclin-dependent kinase complex is a protein complex formed by the association of an inactive catalytic subunit of a protein kinase, cyclin-dependent kinase (CDK), with a regulatory subunit, cyclin. Once cyclin-dependent kinases bind to cyclin, the formed complex is in an activated state. Substrate specificity of the activated complex is mainly established by the associated cyclin within the complex. Activity of CDKCs is controlled by phosphorylation of target proteins, as well as binding of inhibitory proteins.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.
Cyclin A is a member of the cyclin family, a group of proteins that function in regulating progression through the cell cycle. The stages that a cell passes through that culminate in its division and replication are collectively known as the cell cycle Since the successful division and replication of a cell is essential for its survival, the cell cycle is tightly regulated by several components to ensure the efficient and error-free progression through the cell cycle. One such regulatory component is cyclin A which plays a role in the regulation of two different cell cycle stages.

p16, is a protein that slows cell division by slowing the progression of the cell cycle from the G1 phase to the S phase, thereby acting as a tumor suppressor. It is encoded by the CDKN2A gene. A deletion in this gene can result in insufficient or non-functional p16, accelerating the cell cycle and resulting in many types of cancer.
INK4 is a family of cyclin-dependent kinase inhibitors (CKIs). The members of this family (p16INK4a, p15INK4b, p18INK4c, p19INK4d) are inhibitors of CDK4 (hence their name INhibitors of CDK4), and of CDK6. The other family of CKIs, CIP/KIP proteins are capable of inhibiting all CDKs. Enforced expression of INK4 proteins can lead to G1 arrest by promoting redistribution of Cip/Kip proteins and blocking cyclin E-CDK2 activity. In cycling cells, there is a resassortment of Cip/Kip proteins between CDK4/5 and CDK2 as cells progress through G1. Their function, inhibiting CDK4/6, is to block progression of the cell cycle beyond the G1 restriction point. In addition, INK4 proteins play roles in cellular senescence, apoptosis and DNA repair.

Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. The synthesis of cyclin D is initiated during G1 and drives the G1/S phase transition. Cyclin D protein is anywhere from 155 to 477 amino acids in length.

Cyclin-dependent kinase 2, also known as cell division protein kinase 2, or Cdk2, is an enzyme that in humans is encoded by the CDK2 gene. The protein encoded by this gene is a member of the cyclin-dependent kinase family of Ser/Thr protein kinases. This protein kinase is highly similar to the gene products of S. cerevisiae cdc28, and S. pombe cdc2, also known as Cdk1 in humans. It is a catalytic subunit of the cyclin-dependent kinase complex, whose activity is restricted to the G1-S phase of the cell cycle, where cells make proteins necessary for mitosis and replicate their DNA. This protein associates with and is regulated by the regulatory subunits of the complex including cyclin E or A. Cyclin E binds G1 phase Cdk2, which is required for the transition from G1 to S phase while binding with Cyclin A is required to progress through the S phase. Its activity is also regulated by phosphorylation. Multiple alternatively spliced variants and multiple transcription initiation sites of this gene have been reported. The role of this protein in G1-S transition has been recently questioned as cells lacking Cdk2 are reported to have no problem during this transition.

Cell division protein kinase 6 (CDK6) is an enzyme encoded by the CDK6 gene. It is regulated by cyclins, more specifically by Cyclin D proteins and Cyclin-dependent kinase inhibitor proteins. The protein encoded by this gene is a member of the cyclin-dependent kinase, (CDK) family, which includes CDK4. CDK family members are highly similar to the gene products of Saccharomyces cerevisiae cdc28, and Schizosaccharomyces pombe cdc2, and are known to be important regulators of cell cycle progression in the point of regulation named R or restriction point.
CDK7 is a cyclin-dependent kinase shown to be not easily classified. CDK7 is both a CDK-activating kinase (CAK) and a component of the general transcription factor TFIIH.

Cyclin D1 is a protein that in humans is encoded by the CCND1 gene.

G1/S-specific cyclin-D3 is a protein that in humans is encoded by the CCND3 gene.

G1/S-specific cyclin-D2 is a protein that in humans is encoded by the CCND2 gene.

Cyclin-A2 is a protein that in humans is encoded by the CCNA2 gene. It is one of the two types of cyclin A: cyclin A1 is expressed during meiosis and embryogenesis while cyclin A2 is expressed in dividing somatic cells.

Cyclin-dependent kinase 4 inhibitor B also known as multiple tumor suppressor 2 (MTS-2) or p15INK4b is a protein that is encoded by the CDKN2B gene in humans.
Cyclin-dependent kinase 4 inhibitor C is an enzyme that in humans is encoded by the CDKN2C gene.

Cyclin-dependent kinase 4 inhibitor D is an enzyme that in humans is encoded by the CDKN2D gene.

Cell division protein kinase 3 is an enzyme that in humans is encoded by the CDK3 gene.
The CIP/KIP family is one of two families of mammalian cyclin dependent kinase (CDK) inhibitors (CKIs) involved in regulating the cell cycle. The CIP/KIP family is made up of three proteins: p21cip1/waf1, P27kip1, p57kip2 These proteins share sequence homology at the N-terminal domain which allows them to bind to both the cyclin and CDK. Their activity primarily involves the binding and inhibition of G1/S- and S-Cdks; however, they have also been shown to play an important role in activating the G1-CDKs CDK4 and CDK6. In addition, more recent work has shown that CIP/KIP family members have a number of CDK-independent roles involving regulation of transcription, apoptosis, and the cytoskeleton.