E2F family
Schematic diagram of the amino acid sequences of E2F family members (N-terminus to the left, C-terminus to the right) highlighting the relative locations of functional domains within each member:
| Family members | Legend |
|---|---|
 |
E2F is a group of genes that encodes a family of transcription factors (TF) in higher eukaryotes. Three of them are activators: E2F1, 2 and E2F3a. Six others act as suppressors: E2F3b, E2F4-8. All of them are involved in the cell cycle regulation and synthesis of DNA in mammalian cells. E2Fs as TFs bind to the TTTCCCGC (or slight variations of this sequence) consensus binding site in the target promoter sequence.
Schematic diagram of the amino acid sequences of E2F family members (N-terminus to the left, C-terminus to the right) highlighting the relative locations of functional domains within each member:
| Family members | Legend |
|---|---|
 |
Homo sapiens E2F1 mRNA or E2F1 protein sequences from NCBI protein and nucleotide database.
X-ray crystallographic analysis has shown that the E2F family of transcription factors has a fold similar to the winged-helix DNA-binding motif. [1]


E2F family members play a major role during the G1/S transition in mammalian and plant cell cycle (see KEGG cell cycle pathway). DNA microarray analysis reveals unique sets of target promoters among E2F family members suggesting that each protein has a unique role in the cell cycle. [2] Among E2F transcriptional targets are cyclins, CDKs, checkpoints regulators, DNA repair and replication proteins. Nonetheless, there is a great deal of redundancy among the family members. Mouse embryos lacking E2F1, E2F2, and one of the E2F3 isoforms, can develop normally when either E2F3a or E2F3b, is expressed. [3]
The E2F family is generally split by function into two groups: transcription activators and repressors. Activators such as E2F1, E2F2, E2F3a promote and help carryout the cell cycle, while repressors inhibit the cell cycle. Yet, both sets of E2F have similar domains. E2F1-6 have DP1,2 heterodimerization domain which allows them to bind to DP1 or DP2, proteins distantly related to E2F. Binding with DP1,2 provides a second DNA binding site, increasing E2F binding stability. [4] Most E2F have a pocket protein binding domain. Pocket proteins such as pRB and related proteins p107 and p130, can bind to E2F when hypophosphorylated. In activators, E2F binding with pRB has been shown to mask the transactivation domain responsible for transcription activation. [5] In repressors E2F4 and E2F5, pocket protein binding (more often p107 and p130 than pRB) mediates recruitment of repression complexes to silence target genes. [6] E2F6, E2F7, and E2F8 do not have pocket protein binding sites and their mechanism for gene silencing is unclear. Cdk4(6)/cyclin D and cdk2/cyclin E phosphorylate pRB and related pocket proteins allowing them to disassociate from E2F. Activator E2F proteins can then transcribe S phase promoting genes. In REF52 cells, overexpression of activator E2F1 is able to push quiescent cells into S phase. [7] While repressors E2F4 and 5 do not alter cell proliferation, they mediate G1 arrest. [2]
E2F activator levels are cyclic, with maximal expression during G1/S. In contrast, E2F repressors stay constant, especially since they are often expressed in quiescent cells. Specifically, E2F5 is only expressed in terminally differentiated cells in mice. [2] The balance between repressor and activator E2F regulate cell cycle progression. When activator E2F family proteins are knocked out, repressors become active to inhibit E2F target genes. [8]
The Rb tumor suppressor protein (pRb) binds to the E2F1 transcription factor preventing it from interacting with the cell's transcription machinery. In the absence of pRb, E2F1 (along with its binding partner DP1) mediates the trans-activation of E2F1 target genes that facilitate the G1/S transition and S-phase. E2F targets genes that encode proteins involved in DNA replication (for example DNA polymerase, thymidine kinase, dihydrofolate reductase and cdc6), and chromosomal replication (replication origin-binding protein HsOrc1 and MCM5). When cells are not proliferating, E2F DNA binding sites contribute to transcriptional repression. In vivo footprinting experiments obtained on Cdc2 and B-myb promoters demonstrated E2F DNA binding site occupation during G0 and early G1, when E2F is in transcriptional repressive complexes with the pocket proteins.
pRb is one of the targets of the oncogenic human papilloma virus protein E7, and human adenovirus protein E1A. By binding to pRB, they stop the regulation of E2F transcription factors and drive the cell cycle to enable virus genome replication.
Activators are maximally expressed late in G1 and can be found in association with E2F regulated promoters during the G1/S transition. The activation of E2F-3a genes follows upon the growth factor stimulation and the subsequent phosphorylation of the E2F inhibitor retinoblastoma protein, pRB. The phosphorylation of pRB is initiated by cyclin D/cdk4, cdk6 complex and continued by cyclin E/cdk2. Cyclin D/cdk4,6 itself is activated by the MAPK signaling pathway.
When bound to E2F-3a, pRb can directly repress E2F-3a target genes by recruiting chromatin remodeling complexes and histone modifying activities (e.g. histone deacetylase, HDAC) to the promoter.

The cell cycle, or cell-division cycle, is the series of events that take place in a cell that causes it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.

The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult neuronal cells, among the most metabolically active cells in the body, are fully differentiated and reside in a terminal G0 phase. Neurons reside in this state, not because of stochastic or limited nutrient supply, but as a part of their developmental program.

S phase (Synthesis phase) is the phase of the cell cycle in which DNA is replicated, occurring between G1 phase and G2 phase. Since accurate duplication of the genome is critical to successful cell division, the processes that occur during S-phase are tightly regulated and widely conserved.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.
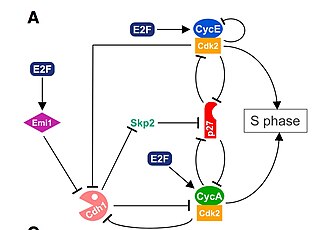
The G1/S transition is a stage in the cell cycle at the boundary between the G1 phase, in which the cell grows, and the S phase, during which DNA is replicated. It is governed by cell cycle checkpoints to ensure cell cycle integrity and the subsequent S phase can pause in response to improperly or partially replicated DNA. During this transition the cell makes decisions to become quiescent, differentiate, make DNA repairs, or proliferate based on environmental cues and molecular signaling inputs. The G1/S transition occurs late in G1 and the absence or improper application of this highly regulated checkpoint can lead to cellular transformation and disease states such as cancer.
Cyclin A is a member of the cyclin family, a group of proteins that function in regulating progression through the cell cycle. The stages that a cell passes through that culminate in its division and replication are collectively known as the cell cycle Since the successful division and replication of a cell is essential for its survival, the cell cycle is tightly regulated by several components to ensure the efficient and error-free progression through the cell cycle. One such regulatory component is cyclin A which plays a role in the regulation of two different cell cycle stages.

Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. The synthesis of cyclin D is initiated during G1 and drives the G1/S phase transition. Cyclin D protein is anywhere from 155 to 477 amino acids in length.
The Cyclin D/Cdk4 complex is a multi-protein structure consisting of the proteins Cyclin D and cyclin-dependent kinase 4, or Cdk4, a serine-threonine kinase. This complex is one of many cyclin/cyclin-dependent kinase complexes that are the "hearts of the cell-cycle control system" and govern the cell cycle and its progression. As its name would suggest, the cyclin-dependent kinase is only active and able to phosphorylate its substrates when it is bound by the corresponding cyclin. The Cyclin D/Cdk4 complex is integral for the progression of the cell from the Growth 1 phase to the Synthesis phase of the cell cycle, for the Start or G1/S checkpoint.
Transcription factor E2F1 is a protein that in humans is encoded by the E2F1 gene.

Retinoblastoma-like protein 2 is a protein that in humans is encoded by the RBL2 gene.

Transcription factor E2F4 is a protein that in humans is encoded by the E2F4 gene.

Transcription factor E2F3 is a protein that in humans is encoded by the E2F3 gene.

Transcription factor E2F2 is a protein that in humans is encoded by the E2F2 gene.

Retinoblastoma-like 1 (p107), also known as RBL1, is a protein that in humans is encoded by the RBL1 gene.

Transcription factor E2F5 is a protein that in humans is encoded by the E2F5 gene.

The retinoblastoma protein is a tumor suppressor protein that is dysfunctional in several major cancers. One function of pRb is to prevent excessive cell growth by inhibiting cell cycle progression until a cell is ready to divide. When the cell is ready to divide, pRb is phosphorylated, inactivating it, and the cell cycle is allowed to progress. It is also a recruiter of several chromatin remodeling enzymes such as methylases and acetylases.
The dimerization partner, RB-like, E2F and multi-vulval class B (DREAM) complex is a protein complex responsible for the regulation of cell cycle-dependent gene expression. The complex is evolutionarily conserved, although some of its components vary from species to species. In humans, the key proteins in the complex are RBL1 (p107) and RBL2 (p130), both of which are homologs of RB (p105) and bind repressive E2F transcription factors E2F4 and E2F5; DP1, DP2 and DP3, dimerization partners of E2F; and MuvB, which is a complex of LIN9/37/52/54 and RBBP4.
The Neuronal cell cycle represents the life cycle of the biological cell, its creation, reproduction and eventual death. The process by which cells divide into two daughter cells is called mitosis. Once these cells are formed they enter G1, the phase in which many of the proteins needed to replicate DNA are made. After G1, the cells enter S phase during which the DNA is replicated. After S, the cell will enter G2 where the proteins required for mitosis to occur are synthesized. Unlike most cell types however, neurons are generally considered incapable of proliferating once they are differentiated, as they are in the adult nervous system. Nevertheless, it remains plausible that neurons may re-enter the cell cycle under certain circumstances. Sympathetic and cortical neurons, for example, try to reactivate the cell cycle when subjected to acute insults such as DNA damage, oxidative stress, and excitotoxicity. This process is referred to as “abortive cell cycle re-entry” because the cells usually die in the G1/S checkpoint before DNA has been replicated.

The Cyclin E/Cdk2 complex is a structure composed of two proteins, cyclin E and cyclin-dependent kinase 2 (Cdk2). Similar to other cyclin/Cdk complexes, the cyclin E/Cdk2 dimer plays a crucial role in regulating the cell cycle, with this specific complex peaking in activity during the G1/S transition. Once the two cyclin and Cdk subunits are joined together, the complex becomes activated and proceeds to phosphorylate and bind to downstream proteins to ultimately promote cell cycle progression. Although cyclin E can bind to other Cdk proteins, its primary binding partner is Cdk2, and the majority of cyclin E activity occurs when it exists as the cyclin E/Cdk2 complex.