
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

Rhodopsin, also known as visual purple, is a light-sensitive receptor protein involved in visual phototransduction. Its name derives from Ancient Greek ῥόδον (rhódon) for "rose", due to its pinkish color, and ὄψις (ópsis) for "sight". Rhodopsin is a biological pigment found in the rods of the retina and is a G-protein-coupled receptor (GPCR). It belongs to a group of photoswitchable opsins. Rhodopsin is extremely sensitive to light, and thus enables vision in low-light conditions. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which rods are more sensitive.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

Rod cells are photoreceptor cells in the retina of the eye that can function in lower light better than the other type of visual photoreceptor, cone cells. Rods are usually found concentrated at the outer edges of the retina and are used in peripheral vision. On average, there are approximately 92 million rod cells in the human retina. Rod cells are more sensitive than cone cells and are almost entirely responsible for night vision. However, rods have little role in color vision, which is the main reason why colors are much less apparent in dim light.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.

Opsins are a group of proteins made light-sensitive via the chromophore retinal found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision.
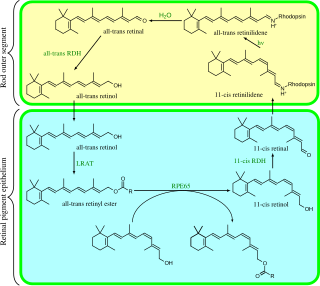
Visual phototransduction is the sensory transduction of the visual system. It is a process by which light is converted into electrical signals in the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye. This cycle was elucidated by George Wald (1906–1997) for which he received the Nobel Prize in 1967. It is so called "Wald's Visual Cycle" after him.

Arrestins are a small family of proteins important for regulating signal transduction at G protein-coupled receptors. Arrestins were first discovered as a part of a conserved two-step mechanism for regulating the activity of G protein-coupled receptors (GPCRs) in the visual rhodopsin system by Hermann Kühn, Scott Hall, and Ursula Wilden and in the β-adrenergic system by Martin J. Lohse and co-workers.

G protein-coupled receptor kinases are a family of protein kinases within the AGC group of kinases. Like all AGC kinases, GRKs use ATP to add phosphate to Serine and Threonine residues in specific locations of target proteins. In particular, GRKs phosphorylate intracellular domains of G protein-coupled receptors (GPCRs). GRKs function in tandem with arrestin proteins to regulate the sensitivity of GPCRs for stimulating downstream heterotrimeric G protein and G protein-independent signaling pathways.

Recoverin is a 23 kilodalton (kDa) neuronal calcium-binding protein that is primarily detected in the photoreceptor cells of the eye. It plays a key role in the inhibition of rhodopsin kinase, a molecule which regulates the phosphorylation of rhodopsin. A reduction in this inhibition helps regulate sensory adaptation in the retina, since the light-dependent channel closure in photoreceptors causes calcium levels to decrease, which relieves the inhibition of rhodopsin kinase by calcium-bound recoverin, leading to a more rapid inactivation of metarhodopsin II.

G-protein-coupled receptor kinase 2 (GRK2) is an enzyme that in humans is encoded by the ADRBK1 gene. GRK2 was initially called Beta-adrenergic receptor kinase, and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK3(βARK2).
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.
Retinylidene proteins, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.

This gene encodes a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinase family, and is most highly similar to GRK4 and GRK5. The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.

G protein-coupled receptor kinase 5 is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases, and is most highly similar to GRK4 and GRK6. The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.

S-arrestin is a protein that in humans is encoded by the SAG gene.

Arrestin-C also known as retinal cone arrestin-3 is a protein that in humans is encoded by the ARR3 gene.

Retinal degeneration is a retinopathy which consists in the deterioration of the retina caused by the progressive death of its cells. There are several reasons for retinal degeneration, including artery or vein occlusion, diabetic retinopathy, R.L.F./R.O.P., or disease. These may present in many different ways such as impaired vision, night blindness, retinal detachment, light sensitivity, tunnel vision, and loss of peripheral vision to total loss of vision. Of the retinal degenerative diseases retinitis pigmentosa (RP) is a very important example.
G-protein-coupled receptor kinase 7 is a serine/threonine-specific protein kinase involved in phototransduction. This enzyme catalyses the phosphorylation of cone (color) photopsins in retinal cones during high acuity color vision primarily in the fovea.

G-protein-coupled receptor kinase 3 (GRK3) is an enzyme that in humans is encoded by the ADRBK2 gene. GRK3 was initially called Beta-adrenergic receptor kinase 2 (βARK-2), and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK2.















