Related Research Articles

Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine. It is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-.
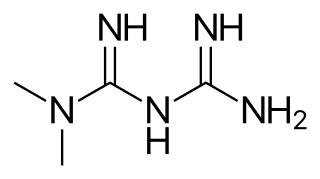
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome. It is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics. Metformin is not associated with weight gain and is taken by mouth.

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.
Ronald Mark Evans is an American Biologist, Professor and Head of the Salk’s Gene Expression Laboratory, and the March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute for Biological Studies in La Jolla, California and a Howard Hughes Medical Institute Investigator. Dr. Ronald M. Evans is known for his original discoveries of nuclear hormone receptors (NR), a special class of transcriptional factor, and the elucidation of their universal mechanism of action, a process that governs how lipophilic hormones and drugs regulate virtually every developmental and metabolic pathway in animals and humans. Nowadays, NRs are among the most widely investigated group of pharmaceutical targets in the world, already yielding benefits in drug discovery for cancer, muscular dystrophies, osteoporosis, type II diabetes, obesity, and cardiovascular diseases. His current research focuses on the function of nuclear hormone signaling and their function in metabolism and cancer.
In oncology, the Warburg effect is the observation that most cancer cells release energy predominantly not through the 'usual' citric acid cycle and oxidative phosphorylation in the mitochondria as observed in normal cells, but through a less efficient process of 'anaerobic glycolysis' consisting of a high level of glucose uptake and glycolysis followed by lactic acid fermentation taking place in the cytosol, not the mitochondria, even in the presence of abundant oxygen. This observation was first published by Otto Heinrich Warburg, who was awarded the 1931 Nobel Prize in Physiology for his "discovery of the nature and mode of action of the respiratory enzyme". The precise mechanism and therapeutic implications of the Warburg effect, however, remain unclear.

Nucleoside-diphosphate kinases are enzymes that catalyze the exchange of terminal phosphate between different nucleoside diphosphates (NDP) and triphosphates (NTP) in a reversible manner to produce nucleotide triphosphates. Many NDP serve as acceptor while NTP are donors of phosphate group. The general reaction via ping-pong mechanism is as follows: XDP + YTP ←→ XTP + YDP. NDPK activities maintain an equilibrium between the concentrations of different nucleoside triphosphates such as, for example, when guanosine triphosphate (GTP) produced in the citric acid (Krebs) cycle is converted to adenosine triphosphate (ATP). Other activities include cell proliferation, differentiation and development, signal transduction, G protein-coupled receptor, endocytosis, and gene expression.
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.

The tumor suppressor gene FLCN encodes the protein folliculin, also known as Birt–Hogg–Dubé syndrome protein, which functions as an inhibitor of Lactate Dehydrogenase-A and a regulator of the Warburg effect. Folliculin (FLCN) is also associated with Birt–Hogg–Dubé syndrome, which is an autosomal dominant inherited cancer syndrome in which affected individuals are at risk for the development of benign cutaneous tumors (folliculomas), pulmonary cysts, and kidney tumors.

Tuberous sclerosis complex 2 (TSC2), also known as tuberin, is a protein that in humans is encoded by the TSC2 gene.

Serine/threonine kinase 11 (STK11) also known as liver kinase B1 (LKB1) or renal carcinoma antigen NY-REN-19 is a protein kinase that in humans is encoded by the STK11 gene.

5'-AMP-activated protein kinase catalytic subunit alpha-1 is an enzyme that in humans is encoded by the PRKAA1 gene.

Galectin-9 was first isolated from mouse embryonic kidney in 1997 as a 36 kDa beta-galactoside lectin protein. Human galectin-9 is encoded by the LGALS9 gene.

NUAK family SNF1-like kinase 1 also known as AMPK-related protein kinase 5 (ARK5) is an enzyme that in humans is encoded by the NUAK1 gene.

Pyruvate kinase isozymes M1/M2 (PKM1/M2), also known as pyruvate kinase muscle isozyme (PKM), pyruvate kinase type K, cytosolic thyroid hormone-binding protein (CTHBP), thyroid hormone-binding protein 1 (THBP1), or opa-interacting protein 3 (OIP3), is an enzyme that in humans is encoded by the PKM2 gene.
Lewis C. Cantley is an American cell biologist and biochemist who has made significant advances to the understanding of cancer metabolism. Among his most notable contributions are the discovery and study of the enzyme PI-3-kinase, now known to be important to understanding cancer and diabetes mellitus. He is currently Meyer Director and Professor of Cancer Biology at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine in New York City. He was formerly a professor in the Departments of Systems Biology and Medicine at Harvard Medical School, and the Director of Cancer Research at the Beth Israel Deaconess Medical Center, in Boston, Massachusetts. In 2016, he was elected Chairman of the Board for the Hope Funds for Cancer Research.
Edelfosine is a synthetic alkyl-lysophospholipid (ALP). It has antineoplastic (anti-cancer) effects.

mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis.

Anthony Rex Hunter is a British-American biologist who is a professor of biology at the Salk Institute for Biological Studies and the University of California San Diego. His research publications list his name as Tony Hunter.
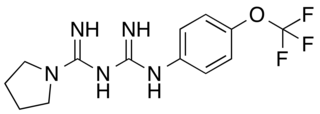
HL156A is a derivative of metformin and a potent oxidative phosphorylation inhibitor and AMP-activated protein kinase activating biguanide. Certain types of cancer cells requires oxidative phosphorylation to survive. By targeting it, HL156A might help in improving anticancer therapy. It is more potent than acadesine or metformin at activating AMP-activated protein kinase. It is synthesized by Hanall Biopharma.
References
- 1 2 "Reuben Shaw, PhD". Salk Institute for Biological Studies. Retrieved 2023-03-13.
- ↑ "Cancer Center Leadership". Salk Institute for Biological Studies. Retrieved 2023-03-13.
- ↑ "NCI-Designated Cancer Centers - NCI". www.cancer.gov. 2012-04-05. Retrieved 2023-08-08.
- 1 2 Shackelford, David B.; Shaw, Reuben J. (2009-08-01). "The LKB1-AMPK pathway: metabolism and growth control in tumour suppression". Nature Reviews. Cancer. 9 (8): 563–575. doi:10.1038/nrc2676. ISSN 1474-1768. PMC 2756045 . PMID 19629071.
- ↑ Meiling, Brittany (2016-01-06). "Reuben Shaw Tapped to Lead Salk Cancer Center". San Diego Business Journal. Retrieved 2023-03-13.
- ↑ "Katja Lamia, Reuben Shaw". The New York Times. 2006-08-20. ISSN 0362-4331 . Retrieved 2023-03-13.
- 1 2 3 "Reuben Shaw: A fated pathway". The Scientist Magazine®. Retrieved 2023-03-13.
- ↑ Shaw, Reuben J.; Kosmatka, Monica; Bardeesy, Nabeel; Hurley, Rebecca L.; Witters, Lee A.; DePinho, Ronald A.; Cantley, Lewis C. (2004-03-09). "The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress". Proceedings of the National Academy of Sciences of the United States of America. 101 (10): 3329–3335. Bibcode:2004PNAS..101.3329S. doi: 10.1073/pnas.0308061100 . ISSN 0027-8424. PMC 373461 . PMID 14985505.
- 1 2 3 "Reuben Shaw, Ph.D., a geneticist and researcher at the Salk Institute: Metabolism Influences Cancer". Leaders in Pharmaceutical Business Intelligence (LPBI) Group. 2014-01-08. Retrieved 2023-03-13.
- ↑ Shaw, Reuben J. (2006-12-01). "Glucose metabolism and cancer". Current Opinion in Cell Biology. 18 (6): 598–608. doi:10.1016/j.ceb.2006.10.005. ISSN 0955-0674. PMID 17046224.
- ↑ Worsley, Oliver (2016-04-27). "Salk team IDs cell 'fuel gauge,' drawing important link in cancer and diabetes metabolism". FierceBiotech. Retrieved 2023-03-13.
- ↑ Goodwin, Jonathan M. (2014-07-17). "A novel AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential". Molecular Cell. 55 (3): 436–450. doi:10.1016/j.molcel.2014.06.021. PMC 4151130 . PMID 25042806.
- ↑ Shaw, Reuben J.; Lamia, Katja A.; Vasquez, Debbie; Koo, Seung-Hoi; Bardeesy, Nabeel; Depinho, Ronald A.; Montminy, Marc; Cantley, Lewis C. (2005-12-09). "The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin". Science. 310 (5754): 1642–1646. Bibcode:2005Sci...310.1642S. doi:10.1126/science.1120781. ISSN 1095-9203. PMC 3074427 . PMID 16308421.
- ↑ "Diabetes drug has unexpected, broad implications for healthy aging: Scientists discover multiple mechanisms at work in widely-used diabetes drug metformin". ScienceDaily. Retrieved 2023-03-13.
- 1 2 "Reuben Shaw, Ph.D., a geneticist and researcher at the Salk Institute: Metabolism Influences Cancer". Leaders in Pharmaceutical Business Intelligence (LPBI) Group. 2014-01-08. Retrieved 2023-03-13.
- ↑ "Identification of Metabolic Targets and Vulnerabilities in Genetically-Defined Cohorts of Lung Cancer". The Mark Foundation for Cancer Research. 2022-03-07. Retrieved 2023-03-13.
- ↑ Shaw, Reuben. "Decoding And Targeting The LKB1-AMPK Signaling Pathway In Cancer".
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Highly Cited Researchers". Clarivate. Retrieved 2023-03-14.
- ↑ "Past lists". Clarivate. Retrieved 2023-03-14.
- ↑ "Lustgarten Foundation awards $5 million for pancreatic cancer research". Philanthropy News Digest. 2022-02-20. Retrieved 2023-03-13.
- ↑ "Inspiring Collaboration in Cancer Research: The Mark Foundation for Cancer Research Announces 2022 Endeavor Award Recipients". The Mark Foundation for Cancer Research. 2022-03-10. Retrieved 2023-03-16.
- ↑ "Curebound | Who We Are". www.curebound.org. Retrieved 2023-08-04.
- ↑ The ASCO Post Staff (August 25, 2017). "Reuben Shaw, PhD, Receives NCI Outstanding Investigator Award - The ASCO Post". ascopost.com. Retrieved 2023-03-13.
- ↑ "Howard Hughes Medical Institute | 2011 Annual Report | Researchers". media.hhmi.org. Retrieved 2023-08-04.
- ↑ "Our Cancer Research Grants". V Foundation. Retrieved 2023-03-14.