
Wilkinson's catalyst (chloridotris(triphenylphosphene)rhodium(I)) is a coordination complex of rhodium with the formula [RhCl(PPh3)3], where 'Ph' denotes a phenyl group. It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds.
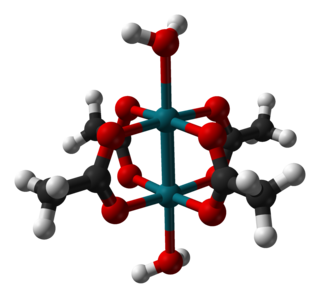
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (CH
3CO−
2). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alkenes. It is a widely studied example of a transition metal carboxylate complex.

Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand, but of course many complexes are known to consist of a mix of aquo and other ligands.
Indium(III) hydroxide is the chemical compound with the formula In(OH)3. Its prime use is as a precursor to indium(III) oxide, In2O3. It is sometimes found as the rare mineral dzhalindite.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
The carbonate chlorides are double salts containing both carbonate and chloride anions. Quite a few minerals are known. Several artificial compounds have been made. Some complexes have both carbonate and chloride ligands. They are part of the family of halocarbonates. In turn these halocarbonates are a part of mixed anion materials.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.
Nitrate chlorides are mixed anion compounds that contain both nitrate (NO3−) and chloride (Cl−) ions. Various compounds are known, including amino acid salts, and also complexes from iron group, rare-earth, and actinide metals. Complexes are not usually identified as nitrate chlorides, and would be termed chlorido nitrato complexes.
The borophosphates are mixed anion compounds containing borate and phosphate anions, which may be joined together by a common oxygen atom. Compounds that contain water or hydroxy groups can also be included in the class of compounds.

Rhodium(III) bromide refers to inorganic compounds of the formula RhBr3(H2O)n where n = 0 or approximately three. Both forms are brown solids. The hydrate is soluble in water and lower alcohols. It is used to prepare rhodium bromide complexes. Rhodium bromides are similar to the chlorides, but have attracted little academic or commercial attention.

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:
The carbonate oxalates are mixed anion compounds that contain both carbonate (CO3) and oxalate (C2O4) anions. Most compounds incorporate large trivalent metal ions, such as the rare earth elements. Some carbonate oxalate compounds of variable composition are formed by heating oxalates.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Manganese(III) chloride is the hypothetical inorganic compound with the formula MnCl3.
Cobalt compounds are chemical compounds formed by cobalt with other elements.











