
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers.
Hyponatremia or hyponatraemia is a low concentration of sodium in the blood. It is generally defined as a sodium concentration of less than 135 mmol/L (135 mEq/L), with severe hyponatremia being below 120 mEq/L. Symptoms can be absent, mild or severe. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance. Severe symptoms include confusion, seizures, and coma; death can ensue.

Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5%. This means that every kilogram of seawater has approximately 35 grams (1.2 oz) of dissolved salts. The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F). The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).
Hypercalcemia, also spelled hypercalcaemia, is a high calcium (Ca2+) level in the blood serum. The normal range is 2.1–2.6 mmol/L (8.8–10.7 mg/dL, 4.3–5.2 mEq/L), with levels greater than 2.6 mmol/L defined as hypercalcemia. Those with a mild increase that has developed slowly typically have no symptoms. In those with greater levels or rapid onset, symptoms may include abdominal pain, bone pain, confusion, depression, weakness, kidney stones or an abnormal heart rhythm including cardiac arrest.
Polydipsia is excessive thirst or excess drinking. The word derives from the Greek πολυδίψιος (poludípsios) "very thirsty", which is derived from πολύς + δίψα. Polydipsia is a nonspecific symptom in various medical disorders. It also occurs as an abnormal behaviour in some non-human animals, such as in birds.
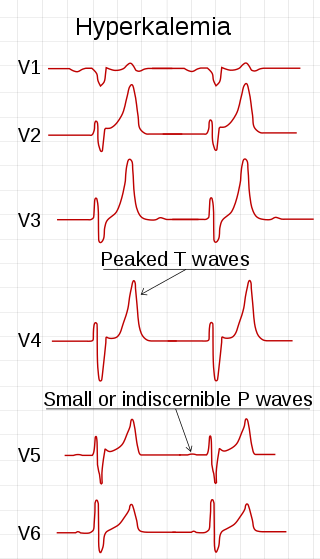
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.

Hypokalemia is a low level of potassium (K+) in the blood serum. Mild low potassium does not typically cause symptoms. Symptoms may include feeling tired, leg cramps, weakness, and constipation. Low potassium also increases the risk of an abnormal heart rhythm, which is often too slow and can cause cardiac arrest.
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood. Early symptoms may include a strong feeling of thirst, weakness, nausea, and loss of appetite. Severe symptoms include confusion, muscle twitching, and bleeding in or around the brain. Normal serum sodium levels are 135–145 mmol/L. Hypernatremia is generally defined as a serum sodium level of more than 145 mmol/L. Severe symptoms typically only occur when levels are above 160 mmol/L.
The syndrome of inappropriate antidiuretic hormone secretion (SIADH), also known as the syndrome of inappropriate antidiuresis (SIAD), is characterized by a physiologically inappropriate release of antidiuretic hormone (ADH) either from the posterior pituitary gland, or an abnormal non-pituitary source. Unsuppressed ADH causes a physiologically inappropriate increase in solute-free water being reabsorbed by the tubules of the kidney to the venous circulation leading to hypotonic hyponatremia.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Water intoxication, also known as water poisoning, hyperhydration, overhydration, water toxemia or hyponatremia is a potentially fatal disturbance in brain functions that can result when the normal balance of electrolytes in the body is pushed outside safe limits by excessive water intake.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.
Sodium ions are necessary in small amounts for some types of plants, but sodium as a nutrient is more generally needed in larger amounts by animals, due to their use of it for generation of nerve impulses and for maintenance of electrolyte balance and fluid balance. In animals, sodium ions are necessary for the aforementioned functions and for heart activity and certain metabolic functions. The health effects of salt reflect what happens when the body has too much or too little sodium. Characteristic concentrations of sodium in model organisms are: 10 mM in E. coli, 30 mM in budding yeast, 10 mM in mammalian cell and 100 mM in blood plasma.

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.

Intravenous sodium bicarbonate, also known as sodium hydrogen carbonate, is a medication primarily used to treat severe metabolic acidosis. For this purpose it is generally only used when the pH is less than 7.1 and when the underlying cause is either diarrhea, vomiting, or the kidneys. Other uses include high blood potassium, tricyclic antidepressant overdose, and cocaine toxicity as well as a number of other poisonings. It is given by injection into a vein.

The health effects of salt are the conditions associated with the consumption of either too much or too little salt. Salt is a mineral composed primarily of sodium chloride (NaCl) and is used in food for both preservation and flavor. Sodium ions are needed in small quantities by most living things, as are chloride ions. Salt is involved in regulating the water content of the body. The sodium ion itself is used for electrical signaling in the nervous system.

Adipsia, also known as hypodipsia, is a symptom of inappropriately decreased or absent feelings of thirst. It involves an increased osmolality or concentration of solute in the urine, which stimulates secretion of antidiuretic hormone (ADH) from the hypothalamus to the kidneys. This causes the person to retain water and ultimately become unable to feel thirst. Due to its rarity, the disorder has not been the subject of many research studies.
Exercise-associated hyponatremia (EAH) is a fluid-electrolyte disorder caused by a decrease in sodium levels (hyponatremia) during or up to 24 hours after prolonged physical activity. This disorder can develop when marathon runners or endurance event athletes drink more fluid, usually water or sports drinks, than their kidneys can excrete. This excess water can severely dilute the level of sodium in the blood needed for organs, especially the brain, to function properly.

Lithium toxicity, also known as lithium overdose, is the condition of having too much lithium. Symptoms may include a tremor, increased reflexes, trouble walking, kidney problems, and an altered level of consciousness. Some symptoms may last for a year after levels return to normal. Complications may include serotonin syndrome.













