The Serine octamer cluster in physical chemistry is an unusually stable cluster consisting of eight serine molecules (Ser) implicated in the origin of homochirality. [1] [2] This cluster was first discovered in mass spectrometry experiments. Electrospray ionization of an aerosol of serine in methanol results in a mass spectrum with a prominent ion peak of 841 corresponding to the Ser8+H+ cation. The smaller and larger clusters are virtually absent in the spectrum and therefore the number 8 is called a magic number. The same octamer ions are also produced by rapid evaporation of a serine solution on a hot (200-250 °C) metal surface or by sublimation of solid serine. After production, detection again is by mass-spectroscopic means. For the discussion of homochirality, these laboratory production methods are designed to mimic prebiotic conditions.
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibrium.
Serine is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.

A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.
The cluster is not only unusually stable but also unusual because the clusters have a strong homochiral preference. A racemic serine solution produces a minimum amount of cluster and with solutions of both enantiomers a maximum amount is formed of both homochiral D-Ser8 and L-Ser8. In another experiment cluster formation of a racemic mixture with deuterium enriched L-serine results in a product distribution with hardly any 50/50 D/L clusters but a preference for either D or L enantioenriched clusters.

In chemistry, an enantiomer, also known as an optical isomer, is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are the same except for being reversed along one axis. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may be some perfect-mirror-image pairs.

Deuterium is one of two stable isotopes of hydrogen. The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutron in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom in 6420 of hydrogen. Thus deuterium accounts for approximately 0.0156% of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another.
A model for chiral amplification is proposed whereby enantioenriched clusters are formed from a non-racemic mixture already enriched by L-serine as a result of a mirror-symmetry breaking process. Cluster formation is followed by isolation and on subsequent dissociation of the cluster a serene solution forms with a higher concentration of L-serine than in the original mixture. A cycle can be maintained in which each turn results in an incremental enrichment in L-serine. Many such cycles eventually result in enantiopure L-serine. This model has been experimentally verified.
Chiral transmission is assumed to take place through so-called substitution reactions of serine clusters. In these reactions, a serine monomer in a cluster can be replaced by another small biologically relevant molecule. For instance Ser8 reacts with glucose (Glc) to the Ser6 + Glc3 + Na+ cluster. Moreover, the cluster of synthetic L-glucose with Ser8 is less abundant than that with the biological D-glucose.
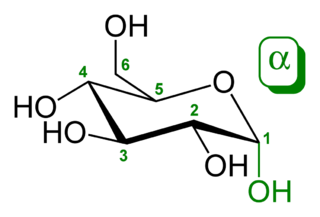
Glucose (also called dextrose) is a simple sugar with the molecular formula C6H12O6. Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. There it is used to make cellulose in cell walls, which is the most abundant carbohydrate. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is partially stored as a polymer, in plants mainly as starch and amylopectin and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is D-glucose, while L-glucose is produced synthetically in comparably small amounts and is of lesser importance.