Related Research Articles

Bacillus Calmette–Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB). It is named after its inventors Albert Calmette and Camille Guérin. In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as soon after birth as possible. In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated. Adults who do not have tuberculosis and have not been previously immunized, but are frequently exposed, may be immunized, as well. BCG also has some effectiveness against Buruli ulcer infection and other nontuberculous mycobacterial infections. Additionally, it is sometimes used as part of the treatment of bladder cancer.

Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating the body's adaptive immunity, they help prevent sickness from an infectious disease. When a sufficiently large percentage of a population has been vaccinated, herd immunity results. Herd immunity protects those who may be immunocompromised and cannot get a vaccine because even a weakened version would harm them. The effectiveness of vaccination has been widely studied and verified. Vaccination is the most effective method of preventing infectious diseases; widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the elimination of diseases such as polio and tetanus from much of the world. However, some diseases, such as measles outbreaks in America, have seen rising cases due to relatively low vaccination rates in the 2010s – attributed, in part, to vaccine hesitancy. According to the World Health Organization, vaccination prevents 3.5–5 million deaths per year.

A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease. The safety and effectiveness of vaccines has been widely studied and verified. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and to further recognize and destroy any of the microorganisms associated with that agent that it may encounter in the future.

Intramuscular injection, often abbreviated IM, is the injection of a substance into a muscle. In medicine, it is one of several methods for parenteral administration of medications. Intramuscular injection may be preferred because muscles have larger and more numerous blood vessels than subcutaneous tissue, leading to faster absorption than subcutaneous or intradermal injections. Medication administered via intramuscular injection is not subject to the first-pass metabolism effect which affects oral medications.
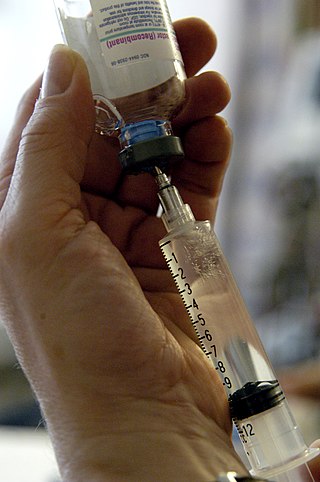
An injection is the act of administering a liquid, especially a drug, into a person's body using a needle and a syringe. An injection is considered a form of parenteral drug administration; it does not involve absorption in the digestive tract. This allows the medication to be absorbed more rapidly and avoid the first pass effect. There are many types of injection, which are generally named after the body tissue the injection is administered into. This includes common injections such as subcutaneous, intramuscular, and intravenous injections, as well as less common injections such as intraperitoneal, intraosseous, intracardiac, intraarticular, and intracavernous injections.

A jet injector is a type of medical injecting syringe device used for a method of drug delivery known as jet injection, in which a narrow, high-pressure stream of liquid penetrates the outermost layer of the skin to deliver medication to targeted underlying tissues of the epidermis or dermis, fat, or muscle.

Vaccine hesitancy is a delay in acceptance, or refusal, of vaccines despite the availability of vaccine services and supporting evidence. The term covers refusals to vaccinate, delaying vaccines, accepting vaccines but remaining uncertain about their use, or using certain vaccines but not others. The scientific consensus that vaccines are generally safe and effective is overwhelming. Vaccine hesitancy often results in disease outbreaks and deaths from vaccine-preventable diseases. Therefore, the World Health Organization characterizes vaccine hesitancy as one of the top ten global health threats.

Fear of needles, known in medical literature as needle phobia, is the extreme fear of medical procedures involving injections or hypodermic needles. This can lead to avoidance of medical care and vaccine hesitancy.
The National Vaccine Information Center (NVIC), founded under the name Dissatisfied Parents Together (DPT) in 1982, is an American 501(c)(3) organization that has been widely criticized as a leading source of fearmongering and misinformation about vaccines. While NVIC describes itself as the "oldest and largest consumer-led organization advocating for the institution of vaccine safety and informed consent protections", it promotes false and misleading information including the discredited claim that vaccines cause autism, and its campaigns portray vaccination as risky, encouraging people to consider "alternatives." In April 2020, the organization was identified as one of the greatest disseminators of COVID-19 misinformation on Facebook.

The National Childhood Vaccine Injury Act (NCVIA) of 1986 was signed into law by United States President Ronald Reagan as part of a larger health bill on November 14, 1986. NCVIA's purpose was to eliminate the potential financial liability of vaccine manufacturers due to vaccine injury claims to ensure a stable market supply of vaccines, and to provide cost-effective arbitration for vaccine injury claims. Under the NCVIA, the National Vaccine Injury Compensation Program (NVICP) was created to provide a federal no-fault system for compensating vaccine-related injuries or death by establishing a claim procedure involving the United States Court of Federal Claims and special masters.

A vaccination schedule is a series of vaccinations, including the timing of all doses, which may be either recommended or compulsory, depending on the country of residence. A vaccine is an antigenic preparation used to produce active immunity to a disease, in order to prevent or reduce the effects of infection by any natural or "wild" pathogen. Vaccines go through multiple phases of trials to ensure safety and effectiveness.
A vaccine adverse event (VAE), sometimes referred to as a vaccine injury, is an adverse event caused by vaccination. The World Health Organization (WHO) knows VAEs as Adverse Events Following Immunization (AEFI).

The Office of Special Masters of the U.S. Court of Federal Claims, popularly known as "vaccine court", administers a no-fault system for litigating vaccine injury claims. These claims against vaccine manufacturers cannot normally be filed in state or federal civil courts, but instead must be heard in the U.S. Court of Federal Claims, sitting without a jury.

A zoster vaccine is a vaccine that reduces the incidence of herpes zoster (shingles), a disease caused by reactivation of the varicella zoster virus, which is also responsible for chickenpox. Shingles provokes a painful rash with blisters, and can be followed by chronic pain, as well as other complications. Older people are more often affected, as are people with weakened immune systems (immunosuppression). Both shingles and postherpetic neuralgia can be prevented by vaccination.
A vaccination policy is a health policy adopted in order to prevent the spread of infectious disease. These policies are generally put into place by State or local governments, but may also be set by private facilities, such as workplaces or schools. Many policies have been developed and implemented since vaccines were first made widely available.
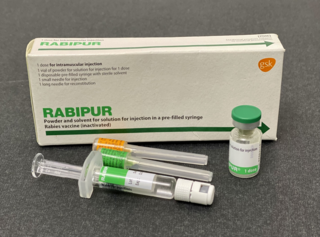
The rabies vaccine is a vaccine used to prevent rabies. There are several rabies vaccines available that are both safe and effective. They can be used to prevent rabies before, and, for a period of time, after exposure to the rabies virus, which is commonly caused by a dog bite or a bat bite.
A Vaccine Information Statement (VIS) is a document designed by the Centers for Disease Control and Prevention (CDC) to provide information to a patient receiving a vaccine in the United States. The National Childhood Vaccine Injury Act requires that medical professionals provide a VIS to patients before receiving certain vaccinations. The VIS includes information about the vaccine's benefits and risks, a description of the vaccine, indications and contraindications, instructions for patients experiencing an adverse reaction, and additional resources.
Anti-vaccinationism in chiropractic is widespread, but there are notable differences within the trade. Chiropractic is a form of alternative medicine founded on the idea that all disease is caused by disruption of the flow of "innate" in the spine, by so-called vertebral subluxations – a pseudoscientific concept. Over time chiropractic has divided into "straights" who adhere to the subluxation theory and "mixers" who adhere more closely to a reality-based view of anatomy. "Straight" chiropractors are very likely to be anti-vaccination, but all chiropractic training tends to reduce acceptance of vaccines.

Vaccination policy of the United States is the subset of U.S. federal health policy that deals with immunization against infectious disease. It is decided at various levels of the government, including the individual states. This policy has been developed over the approximately two centuries since the invention of vaccination with the purpose of eradicating disease from the U.S. population, or creating a herd immunity. Policies intended to encourage vaccination impact numerous areas of law, including regulation of vaccine safety, funding of vaccination programs, vaccine mandates, adverse event reporting requirements, and compensation for injuries asserted to be associated with vaccination.

Vaccine wastage is the number of vaccines that have not been administered during vaccine deployment in an immunization program. The wastage can occur at multiple stages of the deployment process, and can take place in both unopened and opened vials, or in oral admission. It is an expected part of vaccination deployment and is factored into the manufacturing process.
References
- ↑ Brian Dean Abramson, Vaccine, Vaccination, and Immunization Law (Bloomberg Law, 2019), 8-5.
- ↑ VAERS Table of Reportable Events Following Vaccination (as of March 21, 2017).
- 1 2 Bancsi, Ashley; Houle, Sherilyn K.D.; Grindrod, Kelly A. (January 2019). "Shoulder injury related to vaccine administration and other injection site events". Canadian Family Physician. 65 (1): 40–42. ISSN 0008-350X. PMC 6347325 . PMID 30674513.
- ↑ Yuen WL, Loh SY, Wang DB (April 2022). "SIRVA (Shoulder Injury Related to Vaccine Administration) following mRNA COVID-19 Vaccination: Case discussion and literature review". Vaccine. 40 (18): 2546–2550. doi:10.1016/j.vaccine.2022.03.037. PMC 8934720 . PMID 35339304.
- ↑ Chu, Chun-Pu Eric (27 June 2022). "Shoulder Injury Related to Vaccine Administration (SIRVA) in 16 Patients Following COVID-19 Vaccination Who Presented to Chiropractic, Orthopedic, and Physiotherapy Clinics in Hong Kong During 2021". Medical Science Monitor. 28 (e937430): e937430. doi:10.12659/MSM.937430. PMC 9284989 . PMID 35811393 . Retrieved 28 June 2022.
- ↑ Brian Dean Abramson, Vaccine, Vaccination, and Immunization Law (Bloomberg Law, 2019), 9-7.
- ↑ 82 Fed. Reg. 6294 (January 19, 2017).
- ↑ Lindstrom, Rebecca (May 26, 2020). "As labs work on COVID-19 vaccine, government tries to take away right to seek damages if injured by other shots". WXIA-TV . Retrieved July 19, 2020.
- ↑ Wadman, Meredith (April 2, 2020). "United States wants to end most payouts for leading vaccination-related injury". Science.
- ↑ Fleischer, Jodie; Yarborough, Rick; Piper, Jeff (April 20, 2020). "Feds Quietly Seek to Remove Leading Cause of Vaccine Injuries From Federal Payout Program". WRC-TV . Retrieved May 17, 2020.
- ↑ Fleischer, Jodie; Yarborough, Rick; Piper, Jeff (May 20, 2020). "Feds a No-Show When Asked to Prove Why Leading Vaccine Injury Should Be Removed From Compensation Program". WRC-TV . Retrieved July 19, 2020.
- 1 2 "National Vaccine Injury Compensation Program: Revisions to the Vaccine Injury Table". Health and Human Services Department. July 20, 2020.
- ↑ "National Vaccine Injury Compensation Program: Revisions to the Vaccine Injury Table, 86 Fed. Reg. 6249". Health and Human Services Department. January 21, 2021.
- ↑ "National Vaccine Injury Compensation Program: Rescission of Revisions to the Vaccine Injury Table, 86 Fed. Reg. 21209". Health and Human Services Department. April 22, 2021.