Related Research Articles

Poliomyelitis, commonly shortened to polio, is an infectious disease caused by the poliovirus. Approximately 75% of cases are asymptomatic; mild symptoms which can occur include sore throat and fever; in a proportion of cases more severe symptoms develop such as headache, neck stiffness, and paresthesia. These symptoms usually pass within one or two weeks. A less common symptom is permanent paralysis, and possible death in extreme cases. Years after recovery, post-polio syndrome may occur, with a slow development of muscle weakness similar to that which the person had during the initial infection.

A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease. The safety and effectiveness of vaccines has been widely studied and verified. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and recognize further and destroy any of the microorganisms associated with that agent that it may encounter in the future.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.
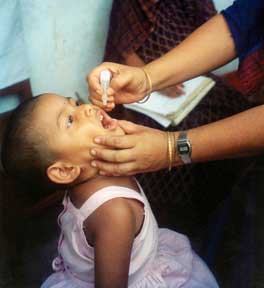
Polio vaccines are vaccines used to prevent poliomyelitis (polio). Two types are used: an inactivated poliovirus given by injection (IPV) and a weakened poliovirus given by mouth (OPV). The World Health Organization (WHO) recommends all children be fully vaccinated against polio. The two vaccines have eliminated polio from most of the world, and reduced the number of cases reported each year from an estimated 350,000 in 1988 to 33 in 2018.

Swine influenza is an infection caused by any of several types of swine influenza viruses. Swine influenza virus (SIV) or swine-origin influenza virus (S-OIV) refers to any strain of the influenza family of viruses that is endemic in pigs. As of 2009, identified SIV strains include influenza C and the subtypes of influenza A known as H1N1, H1N2, H2N1, H3N1, H3N2, and H2N3.

The schedule for childhood immunizations in the United States is published by the Centers for Disease Control and Prevention (CDC). The vaccination schedule is broken down by age: birth to six years of age, seven to eighteen, and adults nineteen and older. Childhood immunizations are key in preventing diseases with epidemic potential.

Contact immunity is the property of some vaccines, where a vaccinated individual can confer immunity upon unimmunized individuals through contact with bodily fluids or excrement. In other words, if person “A” has been vaccinated for virus X and person “B” has not, person “B” can receive immunity to virus X just by coming into contact with person “A”. The term was coined by Romanian physician Ioan Cantacuzino.
Bovine alphaherpesvirus 1 (BoHV-1) is a virus of the family Herpesviridae and the subfamily Alphaherpesvirinae, known to cause several diseases worldwide in cattle, including rhinotracheitis, vaginitis, balanoposthitis, abortion, conjunctivitis, and enteritis. BoHV-1 is also a contributing factor in shipping fever, also known as bovine respiratory disease (BRD). It is spread horizontally through sexual contact, artificial insemination, and aerosol transmission and it may also be transmitted vertically across the placenta. BoHV-1 can cause both clinical and subclinical infections, depending on the virulence of the strain. Although these symptoms are mainly non-life-threatening it is an economically important disease as infection may cause a drop in production and affect trade restrictions. Like other herpesviruses, BoHV-1 causes a lifelong latent infection and sporadic shedding of the virus. The sciatic nerve and trigeminal nerve are the sites of latency. A reactivated latent carrier is normally the source of infection in a herd. The clinical signs displayed are dependent on the virulence of the strain. There is a vaccine available which reduces the severity and incidence of disease. Some countries in Europe have successfully eradicated the disease by applying a strict culling policy.
A booster dose is an extra administration of a vaccine after an earlier (primer) dose. After initial immunization, a booster provides a re-exposure to the immunizing antigen. It is intended to increase immunity against that antigen back to protective levels after memory against that antigen has declined through time. For example, tetanus shot boosters are often recommended every 10 years, by which point memory cells specific against tetanus lose their function or undergo apoptosis.
A breakthrough infection is a case of illness in which a vaccinated individual becomes infected with the illness, because the vaccine has failed to provide complete immunity against the pathogen. Breakthrough infections have been identified in individuals immunized against a variety of diseases including mumps, varicella (Chickenpox), influenza, and COVID-19. The characteristics of the breakthrough infection are dependent on the virus itself. Often, infection of the vaccinated individual results in milder symptoms and shorter duration than if the infection were contracted naturally.

Polio eradication, the permanent global cessation of circulation of the poliovirus and hence elimination of the poliomyelitis (polio) it causes, is the aim of a multinational public health effort begun in 1988, led by the World Health Organization (WHO), the United Nations Children's Fund (UNICEF) and the Rotary Foundation. These organizations, along with the U.S. Centers for Disease Control and Prevention (CDC) and The Gates Foundation, have spearheaded the campaign through the Global Polio Eradication Initiative (GPEI). Successful eradication of infectious diseases has been achieved twice before, with smallpox in humans and rinderpest in ruminants.
Immunization during pregnancy is the administration of a vaccine to a pregnant individual. This may be done either to protect the individual from disease or to induce an antibody response, such that the antibodies cross the placenta and provide passive immunity to the infant after birth. In many countries, including the US, Canada, UK, Australia and New Zealand, vaccination against influenza, COVID-19 and whooping cough is routinely offered during pregnancy.
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen, but still keeping it viable. Attenuation takes an infectious agent and alters it so that it becomes harmless or less virulent. These vaccines contrast to those produced by "killing" the pathogen.
Mass vaccination is a public policy effort to vaccinate a large number of people, possibly the entire population of the world or of a country or region, within a short period of time. This policy may be directed during a pandemic, when there is a localized outbreak or scare of a disease for which a vaccine exists, or when a new vaccine is invented.

Influenza, commonly known as "the flu" or just "flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease.

An inactivated vaccine is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then killed to destroy disease-producing capacity. In contrast, live vaccines use pathogens that are still alive. Pathogens for inactivated vaccines are grown under controlled conditions and are killed as a means to reduce infectivity and thus prevent infection from the vaccine.
DTaP-IPV-HepB vaccine is a combination vaccine whose generic name is diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B (recombinant) and inactivated polio vaccine or DTaP-IPV-Hep B. It protects against the infectious diseases diphtheria, tetanus, pertussis, poliomyelitis, and hepatitis B.
A nasal vaccine is a vaccine administered through the nose that stimulates an immune response without an injection. It induces immunity through the inner surface of the nose, a surface that naturally comes in contact with many airborne microbes. Nasal vaccines are emerging as an alternative to injectable vaccines because they do not use needles and can be introduced through the mucosal route. Nasal vaccines can be delivered through nasal sprays to prevent respiratory infections, such as influenza.
Misinformation related to immunization and the use of vaccines circulates in mass media and social media in spite of the fact that there is no serious hesitancy or debate within mainstream medical and scientific circles about the benefits of vaccination. Unsubstantiated safety concerns related to vaccines are often presented on the internet as being scientific information. A high proportion of internet sources on the topic are "inaccurate on the whole" which can lead people searching for information to form "significant misconceptions about vaccines".
Live recombinant vaccines are biological preparations that stimulate immune responses to a pathogen through the use of genetically modified live bacteria or viruses. These live pathogens are biologically engineered to express exogenous antigens in the cytoplasm of target cells, thereby triggering immune responses. This form of vaccine combines the beneficial features of attenuated and recombinant vaccines, providing the long-lasting immunity of attenuated vaccines’ with recombinant vaccines’ genetically engineered precision and safety.
References
- ↑ Hall CB, Douglas Jr RG, Geiman JM, Meagher MP (1979). "Viral shedding patterns of children with influenza B infection". The Journal of Infectious Diseases. 140 (4): 610–613. doi: 10.1093/infdis/140.4.610 . PMID 512419.
- ↑ Lee B, Kader MA, Colgate ER, Carmolli M, Dickson DM, Diehl SA, Alam M, Afreen S, Mychaleckyj JC, Nayak U, Petri WA, Haque R, Kirkpatrick BD (2021). "Oral rotavirus vaccine shedding as a marker of mucosal immunity". Scientific Reports. Nature. 11 (1): 21760. doi:10.1038/s41598-021-01288-1. PMC 8571310 . PMID 34741103.
- 1 2 3 4 "Debunking the anti-vaccine hoax about 'vaccine shedding'". PolitiFact. Retrieved 2021-05-11.
- 1 2 Tosh PK, Boyce TG, Poland GA (January 2008). "Flu myths: dispelling the myths associated with live attenuated influenza vaccine". Mayo Clinic Proceedings. 83 (1): 77–84. doi: 10.4065/83.1.77 . ISSN 0025-6196. PMID 18174020.
- ↑ "Types of vaccine". Vaccine Knowledge Project. University of Oxford. Retrieved 30 December 2021.
- 1 2 "Vaccines: Breaking down and debunking 10 myths". USA Today. Retrieved 2018-04-29.
- ↑ Johansen N (2021-04-22). "Kelowna store bans anyone who has received COVID-19 vaccine". Castanet. Retrieved 2021-05-11.
- ↑ Hannon E (2021-04-27). "Miami Private School Informs Parents Vaccinated Teachers "May Be Transmitting Something From Their Bodies"". Slate Magazine. Retrieved 2021-05-08.
- ↑ Mazzei P (2021-04-26). "A private school in Miami, citing false claims, bars vaccinated teachers from contact with students". The New York Times. ISSN 0362-4331 . Retrieved 2021-05-11.
- 1 2 "The Latest Anti-Vax Myth: 'Vaccine Shedding'". MedPage Today. 2021-04-29. Retrieved 2021-05-11.
- ↑ "COVID-19 vaccines, irregular periods and spike protein shedding". Nebraska Medicine. 24 April 2021. Retrieved 30 December 2021.
- ↑ "Myths and Facts about COVID-19 Vaccines". Atlanta, Ga.: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. 4 October 2021.
- ↑ "COVID 19 Vaccine Tracker". World Health Organization. Retrieved 30 December 2021.
- 1 2 "No, There's Absolutely Zero Chance of 'Vaccine Shedding' From the COVID-19 Vaccines-Here's Why". Health.com. Retrieved 2021-06-07.
- ↑ Gregory J (13 September 2021). "The Top COVID-19 Vaccine Myths Spreading Online". Encyclopedia Britannica. NewsGuard.
- 1 2 "Polio Vaccine: Vaccine-Derived Poliovirus | CDC". 25 March 2021.
- ↑ Nathanson N, Martin J (1979). "The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance". Am J Epidemiol. 110 (6): 672–92. doi:10.1093/oxfordjournals.aje.a112848. PMID 400274.
- ↑ "International travel and health: Poliomyelitis (Polio)". World Health Organization (WHO). Archived from the original on 24 June 2018. Retrieved 7 July 2018.
- ↑ "Polio Vaccination Causes More Infections than Wild Virus".
- ↑ "Who Should not Get Vaccinated". www.cdc.gov. 2018-03-28. Retrieved 2018-04-29.
- ↑ "Ask the Experts about Rotavirus Vaccines – CDC experts answer Q&As". www.immunize.org. Retrieved 2018-04-29.
- ↑ Anderson EJ (October 2008). "Rotavirus vaccines: viral shedding and risk of transmission". The Lancet Infectious Diseases. 8 (10): 642–649. doi:10.1016/s1473-3099(08)70231-7. ISSN 1473-3099. PMID 18922486.
- ↑ King JC, Treanor J, Fast PE, Wolff M, Yan L, Iacuzio D, Readmond B, O'Brien D, Mallon K (2000-02-01). "Comparison of the Safety, Vaccine Virus Shedding, and Immunogenicity of Influenza Virus Vaccine, Trivalent, Types A and B, Live Cold-Adapted, Administered to Human Immunodeficiency Virus (HIV)-Infected and Non-HIV-Infected Adults". The Journal of Infectious Diseases. 181 (2): 725–728. doi: 10.1086/315246 . ISSN 0022-1899. PMID 10669363.
- ↑ "vaccine-shedding – The Immunization Partnership". www.immunizeusa.org. Archived from the original on 2018-10-07. Retrieved 2018-04-29.
- ↑ "Childhood flu programme: information for healthcare practitioners". GOV.UK. Retrieved 2018-04-29.
- ↑ Calatayud O, Esperón F, Cleaveland S, Biek R, Keyyu J, Eblate E, Neves E, Lembo T, Lankester F (14 June 2019). "Carnivore Parvovirus Ecology in the Serengeti Ecosystem: Vaccine Strains Circulating and New Host Species Identified". Journal of Virology. 93 (13): e02220-18. doi:10.1128/JVI.02220-18. PMC 6580958 . PMID 30996096.
- ↑ "Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products; Guidance for Industry | FDA". www.fda.gov. Retrieved 2021-01-11.
- ↑ Longhurst S. "Current Regulatory Thinking for Viral Shedding Studies in the European Union" (PDF). European Medicines Agency. Retrieved 11 January 2021.
- ↑ "Safety of Influenza Vaccines – Safety of Inactivated Influenza Vaccines (IIVs)". www.cdc.gov. 24 August 2017. Retrieved 3 February 2021.