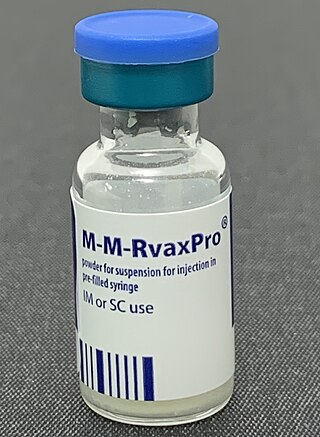
The MMR vaccine is a vaccine against measles, mumps, and rubella, abbreviated as MMR. The first dose is generally given to children around 9 months to 15 months of age, with a second dose at 15 months to 6 years of age, with at least four weeks between the doses. After two doses, 97% of people are protected against measles, 88% against mumps, and at least 97% against rubella. The vaccine is also recommended for those who do not have evidence of immunity, those with well-controlled HIV/AIDS, and within 72 hours of exposure to measles among those who are incompletely immunized. It is given by injection.

Polio vaccines are vaccines used to prevent poliomyelitis (polio). Two types are used: an inactivated poliovirus given by injection (IPV) and a weakened poliovirus given by mouth (OPV). The World Health Organization (WHO) recommends all children be fully vaccinated against polio. The two vaccines have eliminated polio from most of the world, and reduced the number of cases reported each year from an estimated 350,000 in 1988 to 33 in 2018.

Influenza vaccines, also known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research.
The Hong Kong flu, also known as the 1968 flu pandemic, was a flu pandemic whose outbreak in 1968 and 1969 killed between one and four million people globally. It is among the deadliest pandemics in history, and was caused by an H3N2 strain of the influenza A virus. The virus was descended from H2N2 through antigenic shift, a genetic process in which genes from multiple subtypes are reassorted to form a new virus.

Japanese encephalitis (JE) is an infection of the brain caused by the Japanese encephalitis virus (JEV). While most infections result in little or no symptoms, occasional inflammation of the brain occurs. In these cases, symptoms may include headache, vomiting, fever, confusion and seizures. This occurs about 5 to 15 days after infection.

Pneumococcal polysaccharide vaccine, sold under the brand name Pneumovax 23, is a pneumococcal vaccine that is used for the prevention of pneumococcal disease caused by the 23 serotypes of Streptococcus pneumoniae contained in the vaccine as capsular polysaccharides. It is given by intramuscular or subcutaneous injection.

Mumps vaccines are vaccines which prevent mumps. When given to a majority of the population they decrease complications at the population level. Effectiveness when 90% of a population is vaccinated is estimated at 85%. Two doses are required for long term prevention. The initial dose is recommended between 12 and 18 months of age. The second dose is then typically given between two years and six years of age. Usage after exposure in those not already immune may be useful.
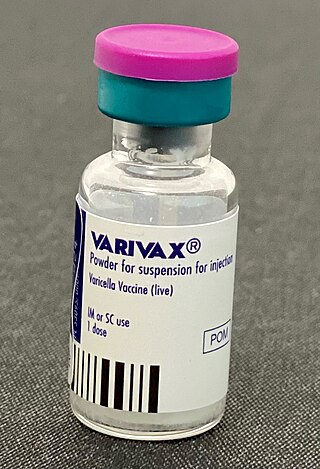
Varicella vaccine, also known as chickenpox vaccine, is a vaccine that protects against chickenpox. One dose of vaccine prevents 95% of moderate disease and 100% of severe disease. Two doses of vaccine are more effective than one. If given to those who are not immune within five days of exposure to chickenpox it prevents most cases of disease. Vaccinating a large portion of the population also protects those who are not vaccinated. It is given by injection just under the skin. Another vaccine, known as zoster vaccine, is used to prevent diseases caused by the same virus – the varicella zoster virus.

Hepatitis B vaccine is a vaccine that prevents hepatitis B. The first dose is recommended within 24 hours of birth with either two or three more doses given after that. This includes those with poor immune function such as from HIV/AIDS and those born premature. It is also recommended that health-care workers be vaccinated. In healthy people, routine immunization results in more than 95% of people being protected.

Hepatitis A vaccine is a vaccine that prevents hepatitis A. It is effective in around 95% of cases and lasts for at least twenty years and possibly a person's entire life. If given, two doses are recommended beginning after the age of one. It is given by injection into a muscle. The first hepatitis A vaccine was approved in Europe in 1991, and the United States in 1995. It is on the World Health Organization's List of Essential Medicines.
The rotavirus vaccine is a vaccine used to protect against rotavirus infections, which are the leading cause of severe diarrhea among young children. The vaccines prevent 15–34% of severe diarrhea in the developing world and 37–96% of the risk of death among young children due to severe diarrhea. Immunizing babies decreases rates of disease among older people and those who have not been immunized.
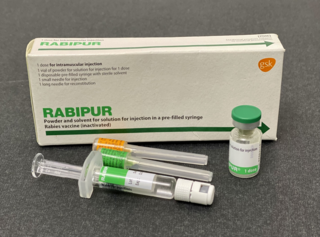
The rabies vaccine is a vaccine used to prevent rabies. There are several rabies vaccines available that are both safe and effective. They can be used to prevent rabies before, and, for a period of time, after exposure to the rabies virus, which is commonly caused by a dog bite or a bat bite.

Diphtheria vaccine is a toxoid vaccine against diphtheria, an illness caused by Corynebacterium diphtheriae. Its use has resulted in a more than 90% decrease in number of cases globally between 1980 and 2000. The first dose is recommended at six weeks of age with two additional doses four weeks apart, after which it is about 95% effective during childhood. Three further doses are recommended during childhood. It is unclear if further doses later in life are needed.

A cholera vaccine is a vaccine that is effective at preventing cholera. For the first six months after vaccination it provides about 85 percent protection, which decreases to 50 percent or 62 percent during the first year. After two years the level of protection decreases to less than 50 percent. When enough of the population is immunized, it may protect those who have not been immunized.

Tick-borne encephalitis vaccine is a vaccine used to prevent tick-borne encephalitis (TBE). The disease is most common in Central and Eastern Europe, and Northern Asia. More than 87% of people who receive the vaccine develop immunity. It is not useful following the bite of an infected tick. It is given by injection into a muscle.

Measles vaccine protects against becoming infected with measles. Nearly all of those who do not develop immunity after a single dose develop it after a second dose. When rate of vaccination within a population is greater than 92%, outbreaks of measles typically no longer occur; however, they may occur again if the rate of vaccination decrease. The vaccine's effectiveness lasts many years. It is unclear if it becomes less effective over time. The vaccine may also protect against measles if given within a couple of days after exposure to measles.

Rubella vaccine is a vaccine used to prevent rubella. Effectiveness begins about two weeks after a single dose and around 95% of people become immune. Countries with high rates of immunization no longer see cases of rubella or congenital rubella syndrome. When there is a low level of childhood immunization in a population it is possible for rates of congenital rubella to increase as more women make it to child-bearing age without either vaccination or exposure to the disease. Therefore, it is important for more than 80% of people to be vaccinated. By introducing rubella containing vaccines, rubella has been eradicated in 81 nations, as of mid-2020.

Yellow fever vaccine is a vaccine that protects against yellow fever. Yellow fever is a viral infection that occurs in Africa and South America. Most people begin to develop immunity within ten days of vaccination and 99% are protected within one month, and this appears to be lifelong. The vaccine can be used to control outbreaks of disease. It is given either by injection into a muscle or just under the skin.
Tetanus vaccine, also known as tetanus toxoid (TT), is a toxoid vaccine used to prevent tetanus. During childhood, five doses are recommended, with a sixth given during adolescence.
Dengue vaccine is a vaccine used to prevent dengue fever in humans. Development of dengue vaccines began in the 1920s, but was hindered by the need to create immunity against all four dengue serotypes. As of 2023, there are two commercially available vaccines, sold under the brand names Dengvaxia and Qdenga.















