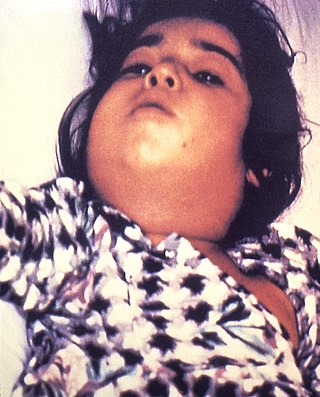
Diphtheria is an infection caused by the bacterium Corynebacterium diphtheriae. Most infections are asymptomatic or have a mild clinical course, but in some outbreaks the lethality rate approaches 10%. Signs and symptoms may vary from mild to severe and usually start two to five days after exposure. Symptoms often develop gradually, beginning with a sore throat and fever. In severe cases, a grey or white patch develops in the throat, which can block the airway and create a barking cough similar to what is observed in croup. The neck may also swell in part due to the enlargement of the facial lymph nodes. Diphtheria can also involve the skin, eyes or genitals, and can cause complications including myocarditis, inflammation of nerves, kidney problems, and bleeding problems due to low levels of platelets.
In biology, immunity is the state of being insusceptible or resistant to a noxious agent or process, especially a pathogen or infectious disease. Immunity may occur naturally or be produced by prior exposure or immunization.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

An antitoxin is an antibody with the ability to neutralize a specific toxin. Antitoxins are produced by certain animals, plants, and bacteria in response to toxin exposure. Although they are most effective in neutralizing toxins, they can also kill bacteria and other microorganisms. Antitoxins are made within organisms, and can be injected into other organisms, including humans, to treat an infectious disease. This procedure involves injecting an animal with a safe amount of a particular toxin. The animal's body then makes the antitoxin needed to neutralize the toxin. Later, blood is withdrawn from the animal. When the antitoxin is obtained from the blood, it is purified and injected into a human or other animal, inducing temporary passive immunity. To prevent serum sickness, it is often best to use an antitoxin obtained from the same species.
ATC code J07Vaccines is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup J07 is part of the anatomical group J Antiinfectives for systemic use.

The DPT vaccine or DTP vaccine is a class of combination vaccines against three infectious diseases in humans: diphtheria, pertussis, and tetanus. The vaccine components include diphtheria and tetanus toxoids and either killed whole cells of the bacterium that causes pertussis or pertussis antigens. The term toxoid refers to vaccines which use an inactivated toxin produced by the pathogen which they are targeted against in order to generate an immune response. In this way, the toxoid vaccine generates an immune response which is targeted against the toxin which is produced by the pathogen and causes disease, rather than a vaccine which is targeted against the pathogen itself. The whole cells or antigens will be depicted as either "DTwP" or "DTaP", where the lower-case "w" indicates whole-cell inactivated pertussis and the lower-case "a" stands for "acellular". In comparison to alternative vaccine types, such as live attenuated vaccines, the DTP vaccine does not contain the pathogen itself, but rather uses inactivated toxoid to generate an immune response; therefore, there is not a risk of use in populations that are immune compromised since there is not any known risk of causing the disease itself. As a result, the DTP vaccine is considered a safe vaccine to use in anyone and it generates a much more targeted immune response specific for the pathogen of interest. However, booster doses are recommended every ten years to maintain immune protection against these pathogens.

A conjugate vaccine is a type of subunit vaccine which combines a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen.
Artificial induction of immunity is immunization achieved by human efforts in preventive healthcare, as opposed to natural immunity as produced by organisms' immune systems. It makes people immune to specific diseases by means other than waiting for them to catch the disease. The purpose is to reduce the risk of death and suffering, that is, the disease burden, even when eradication of the disease is not possible. Vaccination is the chief type of such immunization, greatly reducing the burden of vaccine-preventable diseases.
Passive immunity is the transfer of active humoral immunity of ready-made antibodies. Passive immunity can occur naturally, when maternal antibodies are transferred to the fetus through the placenta, and it can also be induced artificially, when high levels of antibodies specific to a pathogen or toxin are transferred to non-immune persons through blood products that contain antibodies, such as in immunoglobulin therapy or antiserum therapy. Passive immunization is used when there is a high risk of infection and insufficient time for the body to develop its own immune response, or to reduce the symptoms of ongoing or immunosuppressive diseases. Passive immunization can be provided when people cannot synthesize antibodies, and when they have been exposed to a disease that they do not have immunity against.

The Haemophilus influenzae type B vaccine, also known as Hib vaccine, is a vaccine used to prevent Haemophilus influenzae type b (Hib) infection. In countries that include it as a routine vaccine, rates of severe Hib infections have decreased more than 90%. It has therefore resulted in a decrease in the rate of meningitis, pneumonia, and epiglottitis.

Clostridium tetani is a common soil bacterium and the causative agent of tetanus. Vegetative cells of Clostridium tetani are usually rod-shaped and up to 2.5 μm long, but they become enlarged and tennis racket- or drumstick-shaped when forming spores. C. tetani spores are extremely hardy and can be found globally in soil or in the gastrointestinal tract of animals. If inoculated into a wound, C. tetani can grow and produce a potent toxin, tetanospasmin, which interferes with motor neurons, causing tetanus. The toxin's action can be prevented with tetanus toxoid vaccines, which are often administered to children worldwide.

Diphtheria vaccine is a toxoid vaccine against diphtheria, an illness caused by Corynebacterium diphtheriae. Its use has resulted in a more than 90% decrease in number of cases globally between 1980 and 2000. The first dose is recommended at six weeks of age with two additional doses four weeks apart, after which it is about 95% effective during childhood. Three further doses are recommended during childhood. It is unclear if further doses later in life are needed.

Pertussis vaccine is a vaccine that protects against whooping cough (pertussis). There are two main types: whole-cell vaccines and acellular vaccines. The whole-cell vaccine is about 78% effective while the acellular vaccine is 71–85% effective. The effectiveness of the vaccines appears to decrease by between 2 and 10% per year after vaccination with a more rapid decrease with the acellular vaccines. The vaccine is only available in combination with tetanus and diphtheria vaccines. Pertussis vaccine is estimated to have saved over 500,000 lives in 2002.

An inactivated vaccine is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then killed to destroy disease-producing capacity. In contrast, live vaccines use pathogens that are still alive. Pathogens for inactivated vaccines are grown under controlled conditions and are killed as a means to reduce infectivity and thus prevent infection from the vaccine.

Tetanus vaccine, also known as tetanus toxoid (TT), is a toxoid vaccine used to prevent tetanus. During childhood, five doses are recommended, with a sixth given during adolescence.

A hexavalent vaccine, or 6-in-1 vaccine, is a combination vaccine with six individual vaccines conjugated into one, intended to protect people from multiple diseases. The term usually refers to the children's vaccine that protects against diphtheria, tetanus, pertussis, poliomyelitis, haemophilus B, and hepatitis B, which is used in more than 90 countries around the world including in Europe, Canada, Australia, and New Zealand.

A vaccine dose contains many ingredients, very little of which is the active ingredient, the immunogen. A single dose may have merely nanograms of virus particles, or micrograms of bacterial polysaccharides. A vaccine injection, oral drops or nasal spray is mostly water. Other ingredients are added to boost the immune response, to ensure safety or help with storage, and a tiny amount of material is left-over from the manufacturing process. Very rarely, these materials can cause an allergic reaction in people who are very sensitive to them.
Whole-cell vaccines are a type of vaccine that has been prepared in the laboratory in such a way to express immune cells such as cytokines, chemokines and other costimulatory molecules. When administered to the patients, these molecules will stimulate the immune system of the patient. The whole-cell vaccine simultaneously targets multiple antigens to activate the immune system and induces antigen-specific T-cell responses.
Peter Joseph Moloney was a Canadian chemist. He is known for his work on developing vaccines against diphtheria and tetanus, purifying insulin preparations for clinical use, demonstrating antibodies against insulin in humans and animals, and developing sulfated insulin preparations for the treatment of diabetics with insulin resistance. He also invented a quick-acting pH electrode and helped to develop an antiserum that was used in WW II for protection against gas gangrene.














