
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and to further recognize and destroy any of the microorganisms associated with that agent that it may encounter in the future. Vaccines can be prophylactic, or therapeutic. Some vaccines offer full sterilizing immunity, in which infection is prevented completely.

An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals.

Vaccinia virus is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the virion are roughly 360 × 270 × 250 nm, with a mass of approximately 5–10 fg. The vaccinia virus is the source of the modern smallpox vaccine, which the World Health Organisation used to eradicate smallpox in a global vaccination campaign in 1958–1977. Although smallpox no longer exists in the wild, vaccinia virus is still studied widely by scientists as a tool for gene therapy and genetic engineering.

Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.
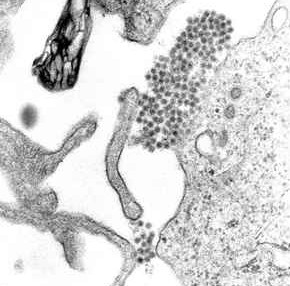
Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, scientists' understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for zika virus and chikungunya complicate matters in real-world infections.

Haemophilus influenzae is a Gram-negative, coccobacillary, facultatively anaerobic capnophilic pathogenic bacterium of the family Pasteurellaceae. H. influenzae was first described in 1892 by Richard Pfeiffer during an influenza pandemic. He incorrectly described Haemophilus influenzae as the causative microbe, which retains "influenza" in its name.

Enterovirus is a genus of positive-sense single-stranded RNA viruses associated with several human and mammalian diseases. Enteroviruses are named by their transmission-route through the intestine.

Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).
Adenovirus infections most commonly cause illness of the respiratory system; however, depending on the infecting serotype, they may also cause various other illnesses and presentations.
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism or in cell culture. Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect. Delivery of genes or other genetic material by a vector is termed transduction and the infected cells are described as transduced. Molecular biologists first harnessed this machinery in the 1970s. Paul Berg used a modified SV40 virus containing DNA from the bacteriophage λ to infect monkey kidney cells maintained in culture.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. The suboptimal antibodies can result from natural infection or from vaccination. ADE may cause enhanced respiratory disease and acute lung injury after respiratory virus infection (ERD) with symptoms of monocytic infiltration and an excess of eosinophils in respiratory tract. ADE along with type 2 T helper cell-dependent mechanisms may contribute to a development of the vaccine associated disease enhancement (VADE), which is not limited to respiratory disease. Some vaccine candidates that targeted coronaviruses, RSV virus and Dengue virus elicited VADE, and were terminated from further development or became approved for use only for patients who had those viruses before.
A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available. Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges. No vaccine is currently available, but several vaccines are currently under development.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. A "subunit" vaccine doesn't contain the whole pathogen, unlike live attenuated or inactivated vaccine, but contains only the antigenic parts such as proteins, polysaccharides or peptides. Because the vaccine doesn't contain "live" components of the pathogen, there is no risk of introducing the disease, and is safer and more stable than vaccine containing whole pathogens. Other advantages include being well-established technology and being suitable for immunocompromised individuals. Disadvantages include being relatively complex to manufacture compared to some vaccines, possibly requiring adjuvants and booster shots, and requiring time to examine which antigenic combinations may work best.
Adenovirus varieties have been explored extensively as a viral vector for gene therapy and also as an oncolytic virus.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

Vaxart, Inc. is an American biotechnology company focused on the discovery, development, and commercialization of oral recombinant vaccines administered using temperature-stable tablets that can be stored and shipped without refrigeration, eliminating the need for needle injection. Its development programs for oral vaccine delivery include prophylactic, enteric-coated tablet vaccines for inhibiting norovirus, seasonal influenza, respiratory syncytial virus, and human papillomavirus. It was founded in 2004 by Sean Tucker.
ChAdOx1 is an adenoviral vector for vaccines that was developed by the Jenner Institute, University of Oxford. The vector is a chimpanzee adenovirus modified to avoid its replication.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material coding for a desired antigen into the recipient's host cells. As of April 2021, six viral vector vaccines have been authorized for use in humans in at least one country: four COVID-19 vaccines and two Ebola vaccines.













