Related Research Articles
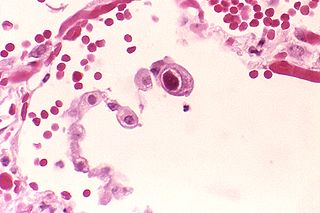
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.
Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B cells may undergo mutations which will render them malignant, giving rise to a lymphoma.

Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat cytomegalovirus (CMV) infections.

Cytomegalovirus retinitis, also known as CMV retinitis, is an inflammation of the retina of the eye that can lead to blindness. Caused by human cytomegalovirus, it occurs predominantly in people whose immune system has been compromised, 15-40% of those with AIDS.

ViroPharma Incorporated was a pharmaceutical company that developed and sold drugs that addressed serious diseases treated by physician specialists and in hospital settings. The company focused on product development activities on viruses and human disease, including those caused by cytomegalovirus (CMV) and hepatitis C virus (HCV) infections. It was purchased by Shire in 2013, with Shire paying around $4.2 billion for the company in a deal that was finalized in January 2014. ViroPharma was a member of the NASDAQ Biotechnology Index and the S&P 600.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.
Correlates of immunity or correlates of protection to a virus or other infectious pathogen are measurable signs that a person is immune, in the sense of being protected against becoming infected and/or developing disease.

Human betaherpesvirus 5, also called human cytomegalovirus (HCMV), is species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.
Sevirumab (MSL-109) is a human monoclonal antibody for the treatment of infections with cytomegalovirus in patients with AIDS.

Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV). Maribavir is a cytomegalovirus pUL97 kinase inhibitor that works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication.

Blueberry muffin baby, also known as extramedullary hematopoiesis, describes a newborn baby with multiple purpura, associated with several non-cancerous and cancerous conditions in which extra blood is produced in the skin. The bumps range from one to seven mm, do not blanch and have a tendency to occur on the head, neck and trunk. They often fade by three to six weeks after birth, leaving brownish marks. When due to a cancer, the bumps tend to be fewer, firmer and larger.
Cytomegalovirus esophagitis is a form of esophagitis associated with cytomegalovirus. Symptoms include dysphagia, upper abdominal pain, diarrhea, nausea, vomiting, and sometimes hematemesis. This condition occurs in the setting of patients with a weakened immune system who are susceptible to both infections by CMV and the manifestation of symptoms. A large majority of patient that have CMV Esophagitis are diagnosed with HIV. Another significant segment of the population have weakened immune systems through transplant surgery, diabetes, or due to medication. Diagnosis is done primarily by endoscopy with biopsy, as CMV Esophagitis has a distinctive pathology pattern of linear ulcers.
Human betaherpesvirus 7 (HHV-7) is one of nine known members of the Herpesviridae family that infects humans. HHV-7 is a member of Betaherpesvirinae, a subfamily of the Herpesviridae that also includes HHV-6 and Cytomegalovirus. HHV-7 often acts together with HHV-6, and the viruses together are sometimes referred to by their genus, Roseolovirus. HHV-7 was first isolated in 1990 from CD4+ T cells taken from peripheral blood lymphocytes.
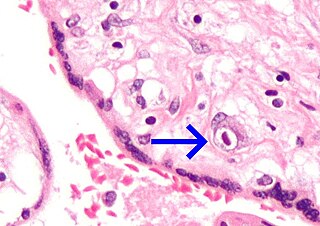
Congenital cytomegalovirus (cCMV) is cytomegalovirus (CMV) infection in a newborn baby. Most have no symptoms. Some affected babies are small. Other signs and symptoms include a rash, jaundice, hepatomegaly, retinitis, and seizures. It may lead to loss of hearing or vision, developmental disability, or a small head.

The term Owl's eye appearance, also known as owl's eye sign, is used to describe a pattern resembling the shape of a real owl's eye that is found in the study of histology, radiology, and pathology cases. The pattern is used to analyze symptoms in patients within the medical field.

Cytomegalovirus colitis, also known as CMV colitis, is an inflammation of the colon.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more virus members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.

Letermovir is an antiviral drug for the treatment of cytomegalovirus (CMV) infections. It has been tested in CMV infected patients with allogeneic stem cell transplants and may also be useful for other patients with a compromised immune system such as those with organ transplants or HIV infections. The drug was initially developed by the anti-infective division at Bayer, which became AiCuris Anti-infective Cures AG through a spin-out and progressed the development to end of Phase 2 before the project was sold to Merck & Co for Phase 3 development and approval.

Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.
Dengue vaccine is a vaccine used to prevent dengue fever in humans. Development of dengue vaccines began in the 1920s, but was hindered by the need to create immunity against all four dengue serotypes. As of 2023, there are two commercially available vaccines, sold under the brand names Dengvaxia and Qdenga.
References
- 1 2 Inoue N, Abe M, Kobayashi R, Yamada S (2018). "Vaccine Development for Cytomegalovirus". Human Herpesviruses. Advances in Experimental Medicine and Biology. Vol. 1045. pp. 271–296. doi:10.1007/978-981-10-7230-7_13. ISBN 978-981-10-7229-1. PMID 29896672.
- 1 2 3 4 Dasari, V.; Smith, C.; Khanna, R. (2013). "Recent advances in designing an effective vaccine to prevent cytomegalovirus-associated clinical diseases". Expert Review of Vaccines. 12 (6): 661–76. doi:10.1586/ERV.13.46. PMID 23750795. S2CID 7062201.
- ↑ Zhong J, Rist M, Cooper L, Smith C, Khanna R (2008). "Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus". PLOS ONE. 3 (9): e3256. Bibcode:2008PLoSO...3.3256Z. doi: 10.1371/journal.pone.0003256 . PMC 2533118 . PMID 18806877.
- ↑ Hajjar, David P.; Schwartz, Stephen M. (1999-02-22). Role of Herpesviruses in Atherogenesis. CRC Press. ISBN 9789057023217.
- ↑ Schleiss MR (March 2008). "Comparison of vaccine strategies against congenital CMV infection in the guinea pig model". J. Clin. Virol. 41 (3): 224–30. doi:10.1016/j.jcv.2007.10.008. PMID 18060834.
- ↑ Schleiss MR, Heineman TC (June 2005). "Progress toward an elusive goal: current status of cytomegalovirus vaccines". Expert Rev Vaccines. 4 (3): 381–406. doi:10.1586/14760584.4.3.381. PMID 16026251. S2CID 5100637.
- ↑ Barnes, Lisa L.; Capuano, Ana W.; Aiello, Alison E.; Turner, Arlener D.; Yolken, Robert H.; Torrey, E. Fuller; Bennett, David A. (2015-01-15). "Cytomegalovirus Infection and Risk of Alzheimer Disease in Older Black and White Individuals". Journal of Infectious Diseases. 211 (2): 230–237. doi:10.1093/infdis/jiu437. ISSN 0022-1899. PMC 4326304 . PMID 25108028.
- ↑ Khanna R, Diamond DJ (January 2006). "Human cytomegalovirus vaccine: time to look for alternative options". Trends Mol Med. 12 (1): 26–33. doi:10.1016/j.molmed.2005.11.006. PMID 16337831.
- ↑ Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R (July 2004). "Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee". Clin. Infect. Dis. 39 (2): 233–9. doi:10.1086/421999. PMID 15307033.
- ↑ Pass RF, Zhang C, Evans A, et al. (2009). "Vaccine prevention of maternal cytomegalovirus infection". N Engl J Med. 360 (12): 1191–9. doi:10.1056/NEJMoa0804749. PMC 2753425 . PMID 19297572.
- ↑ "A Study to Evaluate a Therapeutic Vaccine, ASP0113, in Cytomegalovirus (CMV)-Seropositive Recipients Undergoing Allogeneic, Hematopoietic Cell Transplant (HCT) (HELIOS)". ClinicalTrials.gov. 2013-06-12. Retrieved 2015-10-26.
- ↑ "An Evaluation of a Cytomegalovirus (CMV) Vaccine (ASP0113) in CMV-Seropositive and CMV-Seronegative Healthy Subjects and CMV-Seronegative Dialysis Patients". ClinicalTrials.gov. 2015-07-08. Retrieved 2015-10-22.
- ↑ "Study to Evaluate Safety, Tolerability, and Immunogenicity of Candidate Human Cytomegalovirus Vaccine in Healthy Adults - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 18 September 2016.
- ↑ "Multi-antigen CMV-MVA Triplex Vaccine in Reducing CMV Complications in Patients Previously Infected With CMV and Undergoing Donor Hematopoietic Cell Transplant". ClinicalTrials.gov. 2015-07-21. Retrieved 2016-01-23.
- ↑ "Vaccine Therapy in Reducing the Frequency of Cytomegalovirus Events in Patients With Hematologic Malignancies Undergoing Donor Stem Cell Transplant". ClinicalTrials.gov. 2015-03-12. Retrieved 2016-01-23.
- ↑ Lowe, Derek (21 Apr 2021). "Moderna's Upcoming Clinical Trials". In the Pipeline. American Association for the Advancement of Science. Retrieved 19 October 2021.