Related Research Articles
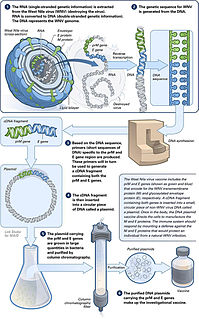
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.
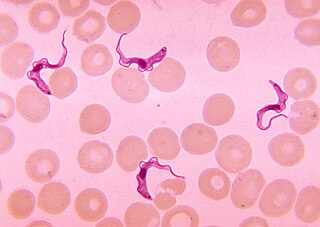
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species Trypanosoma brucei. Humans are infected by two types, Trypanosoma brucei gambiense (TbG) and Trypanosoma brucei rhodesiense (TbR). TbG causes over 98% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas.
In biology, immunity is the capability of multicellular organisms to resist harmful microorganisms. Immunity involves both specific and nonspecific components. The nonspecific components act as barriers or eliminators of a wide range of pathogens irrespective of their antigenic make-up. Other components of the immune system adapt themselves to each new disease encountered and can generate pathogen-specific immunity.

Trypanosoma is a genus of kinetoplastids, a monophyletic group of unicellular parasitic flagellate protozoa. Trypanosoma is part of the phylum Sarcomastigophora. The name is derived from the Greek trypano- (borer) and soma (body) because of their corkscrew-like motion. Most trypanosomes are heteroxenous and most are transmitted via a vector. The majority of species are transmitted by blood-feeding invertebrates, but there are different mechanisms among the varying species. Some, such as Trypanosoma equiperdum, are spread by direct contact. In an invertebrate host they are generally found in the intestine, but normally occupy the bloodstream or an intracellular environment in the vertebrate host.
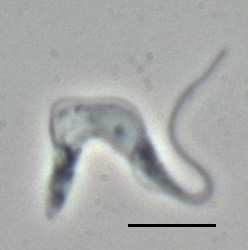
Trypanosoma brucei is a species of parasitic kinetoplastid belonging to the genus Trypanosoma. This parasite is the cause of vector-borne diseases of vertebrate animals, including humans, carried by species of tsetse fly in sub-Saharan Africa. In humans T. brucei causes African trypanosomiasis, or sleeping sickness. In animals it causes animal trypanosomiasis, also called nagana in cattle and horses. T. brucei has traditionally been grouped into three subspecies: T. b. brucei, T. b. gambiense and T. b. rhodesiense. The first is a parasite of non-human vertebrates, while the latter two are known to be parasites of humans. Only rarely can the T. b. brucei infect a human.

Covering sickness, or dourine, is a disease of horses and other members of the family Equidae. The disease is caused by Trypanosoma equiperdum, which belongs to an important genus of parasitic protozoa. The occurrence of dourine is notifiable in the European Union under legislation from the OIE. There currently is no vaccine and although clinical signs can be treated, there is no cure.

Animal trypanosomiasis, also known as nagana and nagana pest, or sleeping sickness, is a disease of vertebrates. The disease is caused by trypanosomes of several species in the genus Trypanosoma such as Trypanosoma brucei. Trypanosoma vivax causes nagana mainly in West Africa, although it has spread to South America. The trypanosomes infect the blood of the vertebrate host, causing fever, weakness, and lethargy, which lead to weight loss and anemia; in some animals the disease is fatal unless treated. The trypanosomes are transmitted by tsetse flies.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response, making it one of the mechanisms of antigenic escape. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.

A malaria vaccine is a vaccine that is used to prevent malaria. The only approved vaccine, as of 2021, is RTS,S, known by the brand name Mosquirix. It requires four injections.
Antigenic escape, immune escape, immune evasion or escape mutation occurs when the immune system of a host, especially of a human being, is unable to respond to an infectious agent, or, in other words, the host's immune system is no longer able to recognize and eliminate a pathogen such as a virus. This process can occur in a number of different ways of both a genetic and an environmental nature. Such mechanisms include homologous recombination, and manipulation and resistance of the host's immune responses.

Trypanosoma congolense is a species of trypanosomes and is the major pathogen responsible for the disease nagana in cattle and other animals including sheep, pigs, goats, horses and camels, dogs, as well as laboratory mice. It is the most common cause of nagana in east Africa, but is also a major cause of nagana in west Africa. This parasite is spread by tsetse flies. In its mammalian host, Trypanosoma congolense only lives in blood vessels, and causes in particular anaemia.
A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available. Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges. No vaccine is currently available, but several vaccines are currently under development.
Cruzipain is a cysteine protease expressed by Trypanosoma cruzi.

Hookworm vaccine is a vaccine against hookworm. No effective vaccine for the disease in humans has yet been developed. Hookworms, parasitic nematodes transmitted in soil, infect approximately 700 million humans, particularly in tropical regions of the world where endemic hookworms include Ancylostoma duodenale and Necator americanus. Hookworms feed on blood and those infected with hookworms may suffer from chronic anaemia and malnutrition. Helminth infection can be effectively treated with benzimidazole drugs, and efforts led by the World Health Organization have focused on one to three yearly de-worming doses in schools because hookworm infections with the heaviest intensities are most common in school-age children. However, these drugs only eliminate existing adult parasites and re-infection can occur soon after treatment. School-based de-worming efforts do not treat adults or pre-school children and concerns exist about drug resistance developing in hookworms against the commonly used treatments, thus a vaccine against hookworm disease is sought to provide more permanent resistance to infection.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy. Immunity due to neutralizing antibodies is also known as sterilizing immunity, as the immune system eliminates the infectious particle before any infection takes place.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most common alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.

RTS,S/AS01 is a recombinant protein-based malaria vaccine. In October 2021, the vaccine was endorsed by the World Health Organization (WHO) for "broad use" in children, making it the first malaria vaccine candidate, and first vaccine to address parasitic infection, to receive this recommendation.

Variant surface glycoprotein (VSG) is a ~60kDa protein which densely packs the cell surface of protozoan parasites belonging to the genus Trypanosoma. This genus is notable for their cell surface proteins. They were first isolated from Trypanosoma brucei in 1975 by George Cross. VSG allows the trypanosomatid parasites to evade the mammalian host's immune system by extensive antigenic variation. They form a 12–15 nm surface coat. VSG dimers, ~90% of all cell surface protein. It also makes up ~10% of total cell protein. For this reason, these proteins are highly immunogenic and an immune response raised against a specific VSG coat will rapidly kill trypanosomes expressing this variant. However, with each cell division there is a possibility that the progeny will switch expression to change the VSG that is being expressed. VSG has no prescribed biochemical activity.
Trypanosoma vivax is a parasite species in the genus Trypanosoma. It causes the disease nagana, also known as animal trypanosomiasis, affecting cattle or wild mammals such as the nyala. It is mainly occurring in West Africa, although it has spread to South America.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material coding for a desired antigen into the recipient's host cells. As of April 2021, six viral vector vaccines have been authorized for use in humans in at least one country: four COVID-19 vaccines and two Ebola vaccines.
References
- ↑ "US Fraunhofer Center receives Grant from the Bill & Melinda Gates Foundation-Intellectual Property" . Retrieved 2009-01-15.
- ↑ Dagenais TR, Demick KP, Bangs JD, Forest KT, Paulnock DM, Mansfield JM (January 2009). "T-Cell Responses to the Trypanosome Variant Surface Glycoprotein Are Not Limited to Hypervariable Subregions". Infect. Immun. 77 (1): 141–51. doi:10.1128/IAI.00729-08. PMC 2612290 . PMID 18936180.
- ↑ Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, Magez S (May 2008). Riley EM (ed.). "Trypanosomiasis-Induced B Cell Apoptosis Results in Loss of Protective Anti-Parasite Antibody Responses and Abolishment of Vaccine-Induced Memory Responses". PLOS Pathog. 4 (5): e1000078. doi:10.1371/journal.ppat.1000078. PMC 2386555 . PMID 18516300.
- ↑ "Trypanosomiasis" . Retrieved 2009-01-15.
- ↑ Lalmanach G, Boulangé A, Serveau C, et al. (May 2002). "Congopain from Trypanosoma congolense: drug target and vaccine candidate". Biol. Chem. 383 (5): 739–49. doi:10.1515/BC.2002.077. PMID 12108538. S2CID 22315392.
- ↑ Authié E, Boulangé A, Muteti D, Lalmanach G, Gauthier F, Musoke AJ (November 2001). "Immunisation of cattle with cysteine proteinases of Trypanosoma congolense: targeting the disease rather than the parasite". Int. J. Parasitol. 31 (13): 1429–33. doi:10.1016/S0020-7519(01)00266-1. PMID 11595229.
- ↑ Autheman, Delphine; Crosnier, Cécile; Clare, Simon; Goulding, David A.; Brandt, Cordelia; Harcourt, Katherine; Tolley, Charlotte; Galaway, Francis; Khushu, Malhar; Ong, Han; Romero-Ramirez, Alessandra (July 2021). "An invariant Trypanosoma vivax vaccine antigen induces protective immunity". Nature. 595 (7865): 96–100. doi: 10.1038/s41586-021-03597-x . ISSN 1476-4687. PMID 34040257.