Related Research Articles

Ethanol is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic drinks, and the second most consumed drug globally behind caffeine.
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated as MeCHO. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
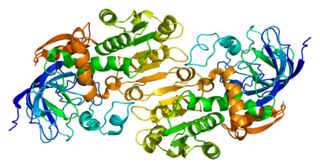
Alcohol dehydrogenases (ADH) (EC 1.1.1.1) are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.

Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol. Disulfiram works by inhibiting the enzyme aldehyde dehydrogenase, causing many of the effects of a hangover to be felt immediately following alcohol consumption. Disulfiram plus alcohol, even small amounts, produces flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, hyperventilation, fast heart rate, low blood pressure, fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe reactions there may be respiratory depression, cardiovascular collapse, abnormal heart rhythms, heart attack, acute congestive heart failure, unconsciousness, convulsions, and death.

Acetaldehyde dehydrogenases are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetyl-CoA. This can be summarized as follows:

1,4-Butanediol, also called Butane-1,4-diol (other names include 1,4-B, BD, BDO and 1,4-BD), is a primary alcohol and an organic compound with the formula HOCH2CH2CH2CH2OH. It is a colorless viscous liquid first synthesized in 1890 via acidic hydrolysis of N,N'-dinitro-1,4-butanediamine by Dutch chemist Pieter Johannes Dekkers, who called it "tetramethylene glycol".

Fomepizole, also known as 4-methylpyrazole, is a medication used to treat methanol and ethylene glycol poisoning. It may be used alone or together with hemodialysis. It is given by injection into a vein.

Alcohol tolerance refers to the bodily responses to the functional effects of ethanol in alcoholic beverages. This includes direct tolerance, speed of recovery from insobriety and resistance to the development of alcohol use disorder.

Silodosin, sold under the brand name Urief among others, is a medication for the symptomatic treatment of benign prostatic hyperplasia. It acts as an alpha-1 adrenergic receptor antagonist.
A hangover is the experience of various unpleasant physiological and psychological effects usually following the consumption of alcohol, such as wine, beer, and liquor. Hangovers can last for several hours or for more than 24 hours. Typical symptoms of a hangover may include headache, drowsiness, concentration problems, dry mouth, dizziness, fatigue, gastrointestinal distress, absence of hunger, light sensitivity, depression, sweating, hyper-excitability, irritability, and anxiety.
In enzymology, an alcohol dehydrogenase (acceptor) (EC 1.1.99.8) is an enzyme that catalyzes the chemical reaction

Alcohol dehydrogenase 1B is an enzyme that in humans is encoded by the ADH1B gene.
Alcohol dehydrogenase (cytochrome c) (EC 1.1.2.8, type I quinoprotein alcohol dehydrogenase, quinoprotein ethanol dehydrogenase) is an enzyme with systematic name alcohol:cytochrome c oxidoreductase. This enzyme catalyses the following chemical reaction
Alcohol dehydrogenase (quinone) (EC 1.1.5.5, type III ADH, membrane associated quinohaemoprotein alcohol dehydrogenase) is an enzyme with systematic name alcohol:quinone oxidoreductase. This enzyme catalyses the following chemical reaction
Alcohol dehydrogenase (azurin) (EC 1.1.9.1, type II quinoprotein alcohol dehydrogenase, quinohaemoprotein ethanol dehydrogenase, QHEDH, ADHIIB) is an enzyme with systematic name alcohol:azurin oxidoreductase. This enzyme catalyses the following chemical reaction

Methanol toxicity is poisoning from methanol, characteristically via ingestion. Symptoms may include a decreased level of consciousness, poor or no coordination, vomiting, abdominal pain, and a specific smell on the breath. Decreased vision may start as early as twelve hours after exposure. Long-term outcomes may include blindness and kidney failure. Blindness may occur after drinking as little as 10 mL; death may occur after drinking quantities over 15 mL.
Alcohol-induced respiratory reactions, also termed alcohol-induced asthma and alcohol-induced respiratory symptoms, are increasingly recognized as a pathological bronchoconstriction response to the consumption of alcohol that afflicts many people with a "classical" form of asthma, the airway constriction disease evoked by the inhalation of allergens. Alcohol-induced respiratory reactions reflect the operation of different and often racially related mechanisms that differ from those of classical, allergen-induced asthma.

Alcohol intolerance is due to a genetic polymorphism of the aldehyde dehydrogenase enzyme, which is responsible for the metabolism of acetaldehyde. This polymorphism is most often reported in patients of East Asian descent. Alcohol intolerance may also be an associated side effect of certain drugs such as disulfiram, metronidazole, or nilutamide. Skin flushing and nasal congestion are the most common symptoms of intolerance after alcohol ingestion. It may also be characterized as intolerance causing hangover symptoms similar to the "disulfiram-like reaction" of aldehyde dehydrogenase deficiency or chronic fatigue syndrome. Severe pain after drinking alcohol may indicate a more serious underlying condition.

Hangover remedies consist of foods, dishes, and medicines, that have been described as having a theoretical potential for easing or alleviating symptoms associated with the hangover.
References
- ↑ David R. Whitmire, US Patent no. 5759539
- ↑ Whitmire, D.; Tedder, J.; Craig, S.; Brown, S. (2008). "The effect of an amethystic product on ethanol in humans". Drug Metabolism and Drug Interactions. 23 (3–4): 283–290. doi:10.1515/DMDI.2008.23.3-4.283. PMID 19326771. S2CID 1719222.
- ↑ "Does Sobrietol really work? We put it to the test". KOMO News. February 6, 2007. Retrieved July 21, 2016.
- ↑ "KWCH - Kansas News and Weather - Sobrietol Put to the Test".
- ↑ "A trick to quick sobriety? TV station says no".
- ↑ "Drink Yourself Sober - News Story - WNEM Saginaw".