Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated as MeCHO. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.

Trimethoprim (TMP) is an antibiotic used mainly in the treatment of bladder infections. Other uses include for middle ear infections and travelers' diarrhea. With sulfamethoxazole or dapsone it may be used for Pneumocystis pneumonia in people with HIV/AIDS. It is taken orally.
This is the timeline of modern antimicrobial (anti-infective) therapy. The years show when a given drug was released onto the pharmaceutical market. This is not a timeline of the development of the antibiotics themselves.

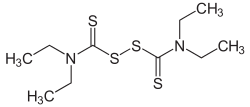
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol. Disulfiram works by inhibiting the enzyme aldehyde dehydrogenase, causing many of the effects of a hangover to be felt immediately following alcohol consumption. Disulfiram plus alcohol, even small amounts, produces flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, hyperventilation, fast heart rate, low blood pressure, fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe reactions there may be respiratory depression, cardiovascular collapse, abnormal heart rhythms, heart attack, acute congestive heart failure, unconsciousness, convulsions, and death.

Metronidazole, sold under the brand name Flagyl among others, is an antibiotic and antiprotozoal medication. It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis. It is effective for dracunculiasis, giardiasis, trichomoniasis, and amebiasis. It is an option for a first episode of mild-to-moderate Clostridioides difficile colitis if vancomycin or fidaxomicin is unavailable. Metronidazole is available orally, as a cream or gel, and by slow intravenous infusion.

Acetaldehyde dehydrogenases are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetyl-CoA. This can be summarized as follows:

The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium.
ATC code J01Antibacterials for systemic use is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup J01 is part of the anatomical group J Antiinfectives for systemic use.

Alcohol flush reaction is a condition in which a person develops flushes or blotches associated with erythema on the face, neck, shoulders, ears, and in some cases, the entire body after consuming alcoholic beverages. The reaction is the result of an accumulation of acetaldehyde, a metabolic byproduct of the catabolic metabolism of alcohol, and is caused by an aldehyde dehydrogenase 2 deficiency.

Tinidazole, sold under the brand name Tindamax among others, is a medication used against protozoan infections. It is widely known throughout Europe and the developing world as a treatment for a variety of anaerobic amoebic and bacterial infections. It was developed in 1972 and is a prominent member of the nitroimidazole antibiotic class.

Fomepizole, also known as 4-methylpyrazole, is a medication used to treat methanol and ethylene glycol poisoning. It may be used alone or together with hemodialysis. It is given by injection into a vein.
Aldehyde dehydrogenases are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes to carboxylic acids. The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes.

Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH2 gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. ALDH2 has a low Km for acetaldehyde, and is localized in mitochondrial matrix. The other liver isozyme, ALDH1, localizes to the cytosol.

Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.

Cefmetazole is a cephamycin antibiotic, usually grouped with the second-generation cephalosporins.

Cefamandole is a second-generation broad-spectrum cephalosporin antibiotic. The clinically used form of cefamandole is the formate ester cefamandole nafate, a prodrug which is administered parenterally. Cefamandole is no longer available in the United States.

Coprine is a mycotoxin. It was first isolated from common inkcap. It occurs in mushrooms in the genera Coprinopsis. When combined with alcohol, it causes "Coprinus syndrome". It inhibits the enzyme acetaldehyde dehydrogenase, which is involved in the metabolism of alcohol. This inhibition leads to a buildup of acetaldehyde, causing an alcohol flush reaction. Because of this, the mushroom is commonly referred to as Tippler's Bane.

Alcohol intolerance is due to a genetic polymorphism of the aldehyde dehydrogenase enzyme, which is responsible for the metabolism of acetaldehyde. This polymorphism is most often reported in patients of East Asian descent. Alcohol intolerance may also be an associated side effect of certain drugs such as disulfiram, metronidazole, or nilutamide. Skin flushing and nasal congestion are the most common symptoms of intolerance after alcohol ingestion. It may also be characterized as intolerance causing hangover symptoms similar to the "disulfiram-like reaction" of aldehyde dehydrogenase deficiency or chronic fatigue syndrome. Severe pain after drinking alcohol may indicate a more serious underlying condition.

Disulfiram-alcohol reaction (DAR) is the effect of the interaction in the human body of alcohol drunk with disulfiram or some mushrooms. The DAR is key to disulfiram therapy that is widely used for alcohol-aversive treatment and management of other addictions. Once disulfiram-treated patients take alcohol, even in small doses, they experience strong unpleasant sensations.
Anti-ulcer agents are medications or supplements used to cure the damage of mucosal layer on organs to prevent the damage from further extending to deeper regions to cause complications.