
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures:
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate * on the surface of the adsorbent(solvent). This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. Adsorption is a surface phenomenon and does not penetrate through the surface to the bulk of the adsorbent, while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term sorption encompasses both processes, while desorption is the reverse of it.
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
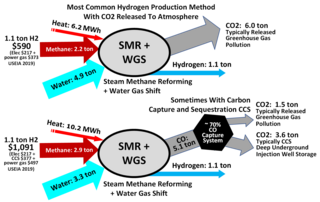
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction.

In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
The water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen:

Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperature and significantly differs from the cryogenic distillation commonly used to separate gases. Selective adsorbent materials are used as trapping material, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed gas.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the carbon dioxide stream that is to be captured can result from burning fossil fuels or biomass. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass plant, and then stored in an underground geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency compared to conventional pulverized coal combustion.
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification, zero-CO2-emission methane pyrolysis, and electrolysis of water. The latter processes, methane pyrolysis as well as water electrolysis can be done directly with any source of electricity, such as solar power.
Douglas Patrick Harrison is a Professor Emeritus of Chemical Engineering from Louisiana State University's Gordon A. and Mary Cain Department of Chemical Engineering, where he taught undergraduate and graduate classes and served as dissertations advisor to Ph.D. and M.S. students. He held the Department Chair position, occupied the Marguerite Voorhies Professor endowed chair, and managed several research projects since his retirement in 2005.

A membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to add reactants or remove products of the reaction.
A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.

Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
A biogas upgrader is a facility that is used to concentrate the methane in biogas to natural gas standards. The system removes carbon dioxide, hydrogen sulphide, water and contaminants from the biogas. One technique for doing this uses amine gas treating. This purified biogas is also called biomethane. It can be used interchangeably with natural gas.
Solid sorbents for carbon capture include a diverse range of porous, solid-phase materials, including mesoporous silicas, zeolites, and metal-organic frameworks. These have the potential to function as more efficient alternatives to amine gas treating processes for selectively removing CO2 from large, stationary sources including power stations. While the technology readiness level of solid adsorbents for carbon capture varies between the research and demonstration levels, solid adsorbents have been demonstrated to be commercially viable for life-support and cryogenic distillation applications. While solid adsorbents suitable for carbon capture and storage are an active area of research within materials science, significant technological and policy obstacles limit the availability of such technologies.
E-diesel is a synthetic diesel fuel created by Audi for use in automobiles. Currently, e-diesel is created by an Audi research facility in partnership with a company named Sunfire. The fuel is created from carbon dioxide, water, and electricity with a process powered by renewable energy sources to create a liquid energy carrier called blue crude which is then refined to generate e-diesel. E-diesel is considered to be a carbon-neutral fuel as it does not extract new carbon and the energy sources to drive the process are from carbon-neutral sources. As of April 2015, an Audi A8 driven by Federal Minister of Education and Research in Germany is using the e-diesel fuel.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.











