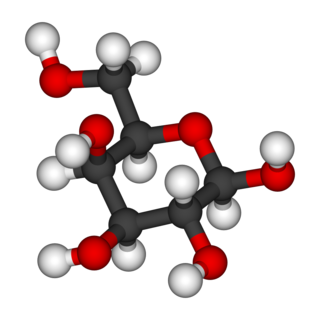
Galactose, sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule.
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets.

Concanavalin A (ConA) is a lectin originally extracted from the jack-bean. It is a member of the legume lectin family. It binds specifically to certain structures found in various sugars, glycoproteins, and glycolipids, mainly internal and nonreducing terminal α-D-mannosyl and α-D-glucosyl groups. Its physiological function in plants, however, is still unknown. ConA is a plant mitogen, and is known for its ability to stimulate mouse T-cell subsets giving rise to four functionally distinct T cell populations, including precursors to regulatory T cells; a subset of human suppressor T-cells is also sensitive to ConA. ConA was the first lectin to be available on a commercial basis, and is widely used in biology and biochemistry to characterize glycoproteins and other sugar-containing entities on the surface of various cells. It is also used to purify glycosylated macromolecules in lectin affinity chromatography, as well as to study immune regulation by various immune cells.

The selectins are a family of cell adhesion molecules. All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers.
The asialoglycoprotein receptors (ASGPR) are lectins which bind asialoglycoprotein and glycoproteins from which a sialic acid has been removed to expose galactose residues. The receptors, which are integral membrane proteins and are located on mammalian hepatocytes, remove target glycoproteins from circulation. The asialoglycoprotein receptor has been demonstrated to have high expression on the surface of hepatocytes and several human carcinoma cell lines It is also weakly expressed by glandular cells of the gallbladder and the stomach. Lactobionic acid has been used as a targeting moiety for drug delivery to cells expressing asialoglycoprotein receptors.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

X-box binding protein 1, also known as XBP1, is a protein which in humans is encoded by the XBP1 gene. The XBP1 gene is located on chromosome 22 while a closely related pseudogene has been identified and localized to chromosome 5. The XBP1 protein is a transcription factor that regulates the expression of genes important to the proper functioning of the immune system and in the cellular stress response.

CD69 is a human transmembrane C-Type lectin protein encoded by the CD69 gene. It is an early activation marker that is expressed in hematopoietic stem cells, T cells, and many other cell types in the immune system. It is also implicated in T cell differentiation as well as lymphocyte retention in lymphoid organs.

Hydroxycarboxylic acid receptor 2 (HCA2), also known as GPR109A and niacin receptor 1 (NIACR1), is a protein which in humans is encoded (its formation is directed) by the HCAR2 gene and in rodents by the Hcar2 gene. The human HCAR2 gene is located on the long (i.e., "q") arm of chromosome 12 at position 24.31 (notated as 12q24.31). Like the two other hydroxycarboxylic acid receptors, HCA1 and HCA3, HCA2 is a G protein-coupled receptor (GPCR) located on the surface membrane of cells. HCA2 binds and thereby is activated by D-β-hydroxybutyric acid (hereafter termed β-hydroxybutyric acid), butyric acid, and niacin (also known as nicotinic acid). β-Hydroxybutyric and butyric acids are regarded as the endogenous agents that activate HCA2. Under normal conditions, niacin's blood levels are too low to do so: it is given as a drug in high doses in order to reach levels that activate HCA2.

Peanut agglutinin (PNA) is plant lectin protein derived from the fruits of Arachis hypogaea. Peanut agglutinin may also be referred to as Arachis hypogaea lectin. Lectins recognise and bind particular sugar sequences in carbohydrates; peanut agglutinin binds the carbohydrate sequence Gal-β(1-3)-GalNAc. The name "peanut agglutinin" originates from its ability to stick together (agglutinate) cells, such as neuraminidase-treated erythrocytes, which have glycoproteins or glycolipids on their surface which include the Gal-β(1-3)-GalNAc carbohydrate sequence.

CD93 is a protein that in humans is encoded by the CD93 gene. CD93 is a C-type lectin transmembrane receptor which plays a role not only in cell–cell adhesion processes but also in host defense.
Galectin-4 is a protein that in humans is encoded by the LGALS4 gene.

Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

C-type lectin domain family 10 member A (CLEC10A) also designated as CD301 is a protein that in humans is encoded by the CLEC10A gene. CLEC10A is part of the C-type lectin superfamily and binds to N-Acetylgalactosamine (GalNAc). It is mainly expressed on myeloid cells and also on oocytes and very early stages of embryogenesis. CLEC10A is used as a marker of the CD1c+ dendritic cell subgroup, also called cDC2. The actions of CLEC10A are diverse, depending on the ligand and environment.
Wheat germ agglutinin (WGA) is a lectin that protects wheat (Triticum) from insects, yeast and bacteria. An agglutinin protein, it binds to N-acetyl-D-glucosamine and sialic acid. WGA has also been shown to interact with sialic acid residues on oligosaccharides. Succinylated WGA is selective for β-N-acetylglucosamine (β-GlcNAc), making it a useful tool for detecting O-GlcNAc. WGA is composed of a mixture of three isoforms, which are quite similar to each other and each contain an unusually high amount of glycine. These three isoforms vary at a total of 10 amino acid positions and all have dimeric structures with four domains per monomer. Each domain is hevein-like and is stabilized by a disulfide bond. N-acetyl-D-glucosamine in the natural environment of wheat is found in the chitin of insects, and the cell membrane of yeast & bacteria. WGA is found abundantly—but not exclusively—in the wheat kernel, where it got the 'germ' name from. In mammals the N-acetyl-D-glucosamine that WGA binds to is found in cartilage and cornea among other places. In those animals sialic acid is found in mucous membranes, e.g. the lining of the inner nose, and digestive tract.

In molecular biology, the jacalin-like lectin domain is a mannose-binding lectin domain with a beta-prism fold consisting of three 4-stranded beta-sheets, with an internal pseudo 3-fold symmetry. Some lectins in this group stimulate distinct T- and B-cell functions, such as Jacalin, which binds to the T-antigen and acts as an agglutinin. This domain is found in 1 to 6 copies in lectins. The domain is also found in the salt-stress induced protein from rice and an animal prostatic spermine-binding protein.

Intelectins are lectins expressed in humans and other chordates. Humans express two types of intelectins encoded by ITLN1 and ITLN2 genes respectively. Several intelectins bind microbe-specific carbohydrate residues. Therefore, intelectins have been proposed to function as immune lectins. Even though intelectins contain fibrinogen-like domain found in the ficolins family of immune lectins, there is significant structural divergence. Thus, intelectins may not function through the same lectin-complement pathway. Most intelectins are still poorly characterized and they may have diverse biological roles. Human intelectin-1 (hIntL-1) has also been shown to bind lactoferrin, but the functional consequence has yet to be elucidated. Additionally, hIntL-1 is a major component of asthmatic mucus and may be involved in insulin physiology as well.
Volkensin is a eukaryotic ribosome-inactivating protein found in the Adenia volkensii plant. It is a glycoprotein with two subunits A and B. A subunit is linked to B subunit with disulfide bridges and non-covalent bonds. B subunit is responsible for binding to the galactosyl-terminated receptors on the cell membrane that allows the entry the A subunit of the toxin into the cell, which performs the inhibitory function. Volkensin is a galactose specific lectin that can inhibit protein synthesis in whole cells and in cell-free lysates. This protein can be included into the category of risin like toxins and it resembles modeccin, the toxin of Adenia digitata. Although very similar in composition, volkensin contains more cysteine residues and more than twice as much sugar than modeccin, due to high content of galactose and mannose. In addition, volkensin is able to inhibit protein synthesis at concentrations 10 times lower than required for modeccin. From gene sequencing analysis, volkensin was found to be coded by 1569-bp ORF, that is 523 amino acid residues without introns. The internal linker sequence is 45 bp. The active site of the A subunit contains Ser203, a novel residue that is conserved in all ribosome inactivating proteins.

Grapiprant, sold under the brand name Galliprant, is a small molecule drug that belongs in the piprant class. This analgesic and anti-inflammatory drug is primarily used as a pain relief for mild to moderate inflammation related to osteoarthritis in dogs. Grapiprant has been approved by the FDA's Center for Veterinary Medicine and was categorized as a non-cyclooxygenase inhibiting non-steroidal anti-inflammatory drug (NSAID) in March 2016.

















