Related Research Articles
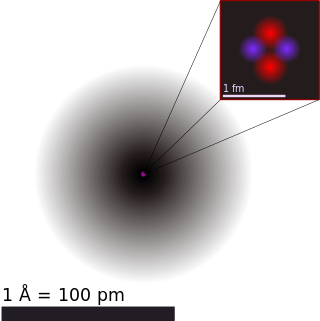
Atomic theory is the scientific theory that matter is composed of particles called atoms. The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the behaviors of gases and how chemical elements reacted with each other. By the end of the 19th century, atomic theory had gained widespread acceptance in the scientific community.

In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. Types of EMR include radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays, all of which are part of the electromagnetic spectrum.

The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.

Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 terahertz, between the infrared and the ultraviolet.

Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO).
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.
Solid-state chemistry, also sometimes referred as materials chemistry, is the study of the synthesis, structure, and properties of solid phase materials. It therefore has a strong overlap with solid-state physics, mineralogy, crystallography, ceramics, metallurgy, thermodynamics, materials science and electronics with a focus on the synthesis of novel materials and their characterization. A diverse range of synthetic techniques, such as the ceramic method and chemical vapour depostion, make solid-state materials. Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles. Their elemental compositions, microstructures, and physcial properties can be characterized through a variety of analytical methods.

Willard Frank Libby was an American physical chemist noted for his role in the 1949 development of radiocarbon dating, a process which revolutionized archaeology and palaeontology. For his contributions to the team that developed this process, Libby was awarded the Nobel Prize in Chemistry in 1960.

Astrophysics is a science that employs the methods and principles of physics and chemistry in the study of astronomical objects and phenomena. As one of the founders of the discipline, James Keeler, said, Astrophysics "seeks to ascertain the nature of the heavenly bodies, rather than their positions or motions in space–what they are, rather than where they are." Among the subjects studied are the Sun, other stars, galaxies, extrasolar planets, the interstellar medium and the cosmic microwave background. Emissions from these objects are examined across all parts of the electromagnetic spectrum, and the properties examined include luminosity, density, temperature, and chemical composition. Because astrophysics is a very broad subject, astrophysicists apply concepts and methods from many disciplines of physics, including classical mechanics, electromagnetism, statistical mechanics, thermodynamics, quantum mechanics, relativity, nuclear and particle physics, and atomic and molecular physics.

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.
The Bakerian Medal is one of the premier medals of the Royal Society that recognizes exceptional and outstanding science. It comes with a medal award and a prize lecture. The medalist is required to give a lecture on any topic related to physical sciences. It is awarded annually to individuals in the field of physical sciences, including computer science.
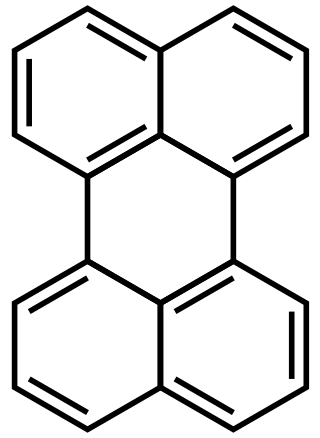
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.
Radiation chemistry is a subdivision of nuclear chemistry which is the study of the chemical effects of radiation on matter; this is very different from radiochemistry as no radioactivity needs to be present in the material which is being chemically changed by the radiation. An example is the conversion of water into hydrogen gas and hydrogen peroxide.
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.
Samuel J. Danishefsky is an American chemist working as a professor at both Columbia University and the Memorial Sloan-Kettering Cancer Center in New York City.
Samuel Colville Lind was a radiation chemist, referred to as "the father of modern radiation chemistry".
The timeline of quantum mechanics is a list of key events in the history of quantum mechanics, quantum field theories and quantum chemistry.
Quantum crystallography is a branch of crystallography that investigates crystalline materials within the framework of quantum mechanics, with analysis and representation, in position or in momentum space, of quantities like wave function, electron charge and spin density, density matrices and all properties related to them. Like the quantum chemistry, Quantum crystallography involves both experimental and computational work. The theoretical part of quantum crystallography is based on quantum mechanical calculations of atomic/molecular/crystal wave functions, density matrices or density models, used to simulate the electronic structure of a crystalline material. While in quantum chemistry, the experimental works mainly rely on spectroscopy, in quantum crystallography the scattering techniques play the central role, although spectroscopy as well as atomic microscopy are also sources of information.
Milton Burton was an American chemist, a pioneer in the field of radiation chemistry and radiobiology. He founded the Radiation Laboratory at University of Notre Dame in 1949 and served as its director from 1963 to 1971. He proposed the G value for describing the chemical yield in radiolytic reactions. He is often referred to as the godfather of radiation chemistry.

John Lafayette Magee was an American chemist known for his work on kinetic models of radiation chemistry, especially the Samuel-Magee model for describing radiolysis in solution.
References
- ↑ Samuel, Aryeh H.; Magee, John L. (1953). "Theory of Radiation Chemistry. II. Track Effects in Radiolysis of Water". The Journal of Chemical Physics. 21 (6): 1080–1087. doi:10.1063/1.1699113. ISSN 0021-9606.
- ↑ Mozumder, A. (1999), "Spur Theory of Radiation Chemical Yields", Fundamentals of Radiation Chemistry, Elsevier, pp. 199–245, doi:10.1016/b978-012509390-3/50007-2, ISBN 978-0-12-509390-3 , retrieved 2022-05-06