Related Research Articles
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.

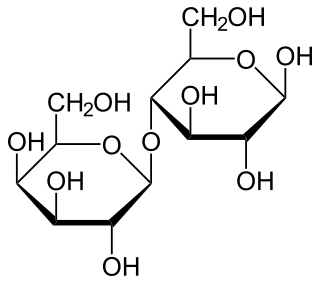
Lactose, or milk sugar, is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from lact (gen. lactis), the Latin word for milk, plus the suffix -ose used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry.

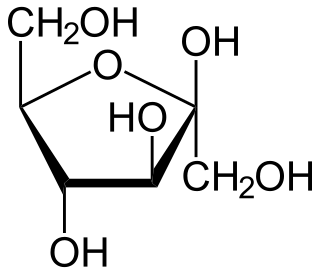
Fructose, or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood.
Lactose intolerance is caused by a lessened ability or a complete inability to digest lactose, a sugar found in dairy products. Humans vary in the amount of lactose they can tolerate before symptoms develop. Symptoms may include abdominal pain, bloating, diarrhea, flatulence, and nausea. These symptoms typically start thirty minutes to two hours after eating or drinking something containing lactose, with the severity typically depending on the amount consumed. Lactose intolerance does not cause damage to the gastrointestinal tract.

Maltose, also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.

Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.
Inverted sugar syrup, also called invert syrup, invert sugar, simple syrup, sugar syrup, sugar water, bar syrup, syrup USP, or sucrose inversion, is a syrup mixture of the monosaccharides glucose and fructose, that is made by hydrolytic saccharification of the disaccharide sucrose. This mixture's optical rotation is opposite to that of the original sugar, which is why it is called an invert sugar.

Hereditary fructose intolerance (HFI) is an inborn error of fructose metabolism caused by a deficiency of the enzyme aldolase B. Individuals affected with HFI are asymptomatic until they ingest fructose, sucrose, or sorbitol. If fructose is ingested, the enzymatic block at aldolase B causes an accumulation of fructose-1-phosphate which, over time, results in the death of liver cells. This accumulation has downstream effects on gluconeogenesis and regeneration of adenosine triphosphate (ATP). Symptoms of HFI include vomiting, convulsions, irritability, poor feeding as a baby, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia and potentially kidney failure. While HFI is not clinically a devastating condition, there are reported deaths in infants and children as a result of the metabolic consequences of HFI. Death in HFI is always associated with problems in diagnosis.

Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its surface area. This facilitates transport of numerous small molecules into the enterocyte from the intestinal lumen. These include broken down proteins, fats, and sugars, as well as water, electrolytes, vitamins, and bile salts. Enterocytes also have an endocrine role, secreting hormones such as leptin.
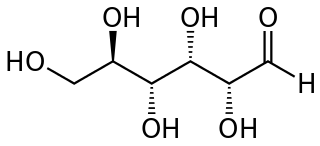
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.
β-Fructofuranosidase is an enzyme that catalyzes the hydrolysis (breakdown) of the table sugar sucrose into fructose and glucose. Alternative names for β-fructofuranosidase EC 3.2.1.26 include invertase, saccharase, glucosucrase, β-fructosidase, invertin, fructosylinvertase, alkaline invertase, acid invertase, and the systematic name: β-fructofuranosidase. The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose. Invertase is a glycoprotein that hydrolyses (cleaves) the non-reducing terminal β-fructofuranoside residues. Invertases cleave the O-C(fructose) bond, whereas the sucrases cleave the O-C(glucose) bond. Invertase cleaves the α-1,2-glycosidic bond of sucrose.
Disaccharidases are glycoside hydrolases, enzymes that break down certain types of sugars called disaccharides into simpler sugars called monosaccharides. In the human body, disaccharidases are made mostly in an area of the small intestine's wall called the brush border, making them members of the group of "brush border enzymes".

Isomaltulose is a disaccharide carbohydrate composed of glucose and fructose. It is naturally present in honey and sugarcane extracts and is also produced industrially from table sugar (sucrose) and used as a sugar alternative.

α-Glucosidase (EC 3.2.1.20, is a glucosidase located in the brush border of the small intestine that acts upon α bonds:

In biochemistry, glycoside hydrolases are a class of enzymes which catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes, with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies, in pathogenesis mechanisms and in normal cellular function. Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds.

Sucrose intolerance or genetic sucrase-isomaltase deficiency (GSID) is the condition in which sucrase-isomaltase, an enzyme needed for proper metabolism of sucrose (sugar) and starch, is not produced or the enzyme produced is either partially functional or non-functional in the small intestine. All GSID patients lack fully functional sucrase, while the isomaltase activity can vary from minimal functionality to almost normal activity. The presence of residual isomaltase activity may explain why some GSID patients are better able to tolerate starch in their diet than others with GSID.

Sucrase-isomaltase is a bifunctional glucosidase located on the brush border of the small intestine, encoded by the human gene SI. It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.

Glucose-galactose malabsorption is a rare condition in which the cells lining the intestine cannot take in the sugars glucose and galactose, which prevents proper digestion of these molecules and larger molecules made from them.

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
Sucrose α-glucosidase is an enzyme with systematic name sucrose-α-D-glucohydrolase. It catalyses the hydrolysis of sucrose and maltose by an α-D-glucosidase-type action.
References
- ↑ Tortora, Gerard (2014). Principles of Anatomy & Physiology 14th edition. USA: Wiley. pp. 924. ISBN 978-1-118-34500-9.
- ↑ Hubert Schiweck; Margaret Clarke; Günter Pollach (2007). "Sugar". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_345.pub2. ISBN 978-3-527-30673-2.
- 1 2 Martínez del Rio, C.; Baker, H. G.; Baker, I. (1992). "Ecological and evolutionary implications of digestive processes: Bird preferences and the sugar constituents of floral nectar and fruit pulp". Experientia . Birkhäuser. 48 (6): 544–551. doi:10.1007/bf01920237. ISSN 0014-4754. S2CID 25707787.