Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Assam tea is a black tea named after the region of its production, Assam, India. It is manufactured specifically from the plant Camellia sinensis var. assamica (Masters). The Assam tea plant is indigenous to Assam—initial efforts to plant the Chinese varieties in Assam soil did not succeed. Assam tea is now mostly grown at or near sea level and is known for its body, briskness, malty flavour, and strong, bright colour. Assam teas, or blends containing Assam tea, are often sold as "breakfast" teas. For instance, Irish breakfast tea, a maltier and stronger breakfast tea, consists of small-sized Assam tea leaves.
The history of tea in China is long and complex, for the Chinese have enjoyed tea for millennia. Scholars hailed the brew as a cure for a variety of ailments; the nobility considered the consumption of good tea as a mark of their status, and the common people simply enjoyed its flavour. In 2016, the discovery of the earliest known physical evidence of tea from the mausoleum of Emperor Jing of Han in Xi'an was announced, indicating that tea from the genus Camellia was drunk by Han dynasty emperors as early as the 2nd century BCE. Tea then became a popular drink in the Tang (618–907) and Song (960–1279) dynasties.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.

Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.

Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position.

Theaflavin-3-gallate is a theaflavin derivative. It can be found in abundance in black tea and is produced during fermentation. It has been studied as a cancer-fighting chemical when combined with cisplatin against ovarian cancer cells. Consuming large amounts of black tea has been reported to reduce the effects of aging in female populations.

Theaflavin digallate (TFDG) is an antioxidant natural phenol found in black tea, and a theaflavin derivative.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Tricetinidin is an intense red-colored chemical compound belonging to the 3-deoxyanthocyanidins. It can be found in black tea infusions. Tricetinidin, in tea, would be a product of the oxidative degallation of epigallocatechin gallate (EGCG).

Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea. It is also reported in buckwheat and in grape.

Gallocatechin gallate (GCG) is the ester of gallocatechin and gallic acid and a type of catechin. It is an epimer of epigallocatechin gallate (EGCG).

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

Instant tea is a powdered mix in which water is added, in order to reconstitute it into a cup of tea. The earliest form of instant tea was developed in the United Kingdom in 1885. A patent was granted for a paste made of concentrated tea extract, sugar, and evaporated milk, which became tea when hot water was added. However, no notable developments were made until spray drying technology allowed for drying the tea concentrates at a temperature which did not damage the flavors of the product.
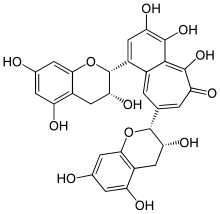
Theasinensin A is polyphenol flavonoid from black tea created during fermentation, by oxidation of epigallocatechin gallate.
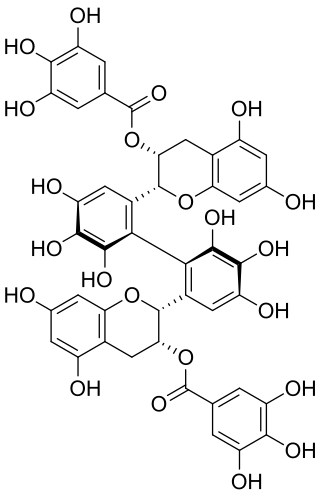
Theasinensin B is polyphenol flavonoid from black tea.

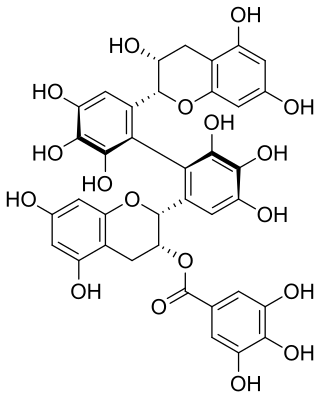
Theasinensin C is polyphenol flavonoid from black tea.