Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.

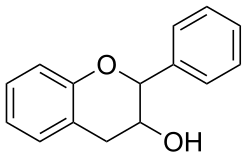
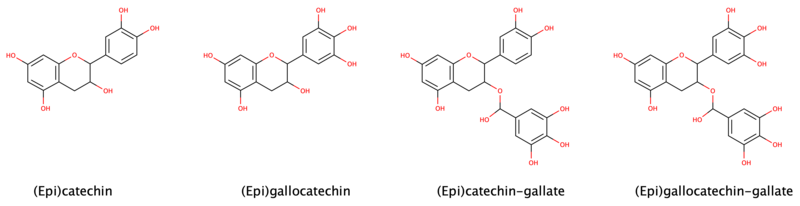
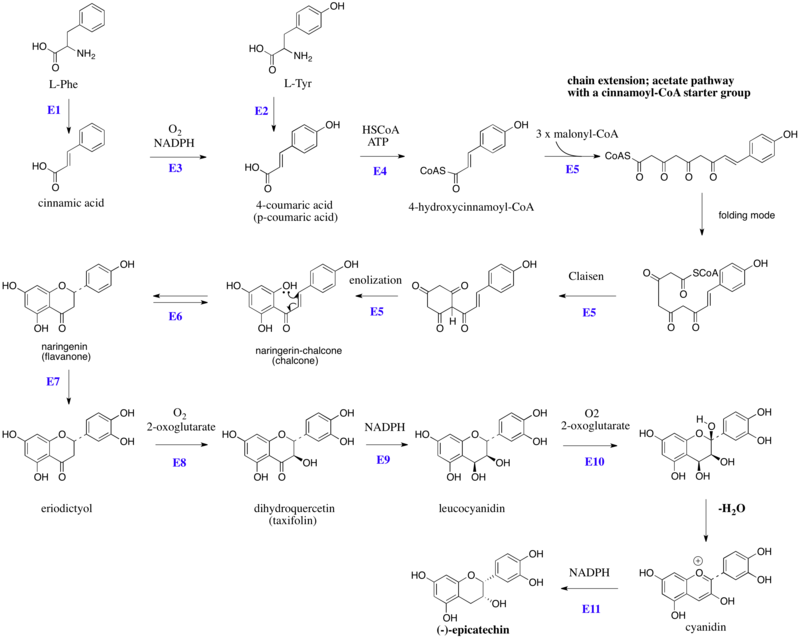
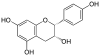
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

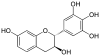
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.

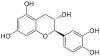
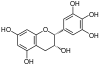
Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.

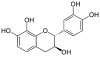
Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea. It is also reported in buckwheat and in grape.

Procyanidin C1 (PCC1) is a B type proanthocyanidin. It is an epicatechin trimer found in grape, unripe apples, and cinnamon.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.

Dark chocolate is a form of chocolate containing only cocoa solids, cocoa butter and sugar. Dark chocolate without added sweetener is known as bitter chocolate or unsweetened chocolate. As with the other two main types of chocolate, dark chocolate is used for chocolate bars or as a coating in confectionery.

Helmut Sies is a German physician, biochemist and university professor. He was the first to demonstrate the existence of hydrogen peroxide as a normal attribute of aerobic life in 1970, and he introduced the concept of Oxidative stress in 1985. He also worked on the biological strategies of antioxidant defense and the biochemistry of nutritional antioxidants.