Endorphins are peptides produced in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in the pituitary gland of the brain. Endorphins are endogenous painkillers often produced in the brain and adrenal medulla during physical exercise or orgasm and inhibit pain, muscle cramps, and relieve stress.
Dynorphins (Dyn) are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing by proprotein convertase 2 (PC2), multiple active peptides are released: dynorphin A, dynorphin B, and α/β-neoendorphin. Depolarization of a neuron containing prodynorphin stimulates PC2 processing, which occurs within synaptic vesicles in the presynaptic terminal. Occasionally, prodynorphin is not fully processed, leading to the release of “big dynorphin.” “Big Dynorphin” is a 32-amino acid molecule consisting of both dynorphin A and dynorphin B.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Opioid peptides or opiate peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, control of food intake, and the rewarding effects of alcohol and nicotine.

Endomorphins are considered to be natural opioid neurotransmitters central to pain relief. The two known endomorphins, endomorphin-1 and endomorphin-2, are tetrapeptides, consisting of Tyr-Pro-Trp-Phe and Tyr-Pro-Phe-Phe amino acid sequences respectively. These sequences fold into tertiary structures with high specificity and affinity for the μ-opioid receptor, binding it exclusively and strongly. Bound μ-opioid receptors typically induce inhibitory effects on neuronal activity. Endomorphin-like immunoreactivity exists within the central and peripheral nervous systems, where endomorphin-1 appears to be concentrated in the brain and upper brainstem, and endomorphin-2 in the spinal cord and lower brainstem. Because endomorphins activate the μ-opioid receptor, which is the target receptor of morphine and its derivatives, endomorphins possess significant potential as analgesics with reduced side effects and risk of addiction.

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.
Dynorphin B, also known as rimorphin, is a form of dynorphin and an endogenous opioid peptide with the amino acid sequence Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr. Dynorphin B is generated as a proteolytic cleavage product of leumorphin, which in turn is a cleavage product of preproenkephalin B (prodynorphin).

Dynorphin A is a dynorphin, an endogenous opioid peptide that activates the κ-opioid receptor. Its amino acid sequence is Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys.
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans. It is one of the two forms of enkephalin; the other is met-enkephalin. The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine. Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.
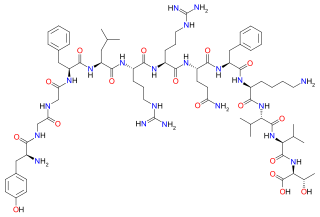
Big dynorphin is an endogenous opioid peptide of the dynorphin family that is composed of both dynorphin A and dynorphin B. Big dynorphin has the amino acid sequence: Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-Lys-Arg-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr. It has nociceptive and anxiolytic-like properties, as well as effects on memory in mice.

Neuropeptides B/W receptor 1, also known as NPBW1 and GPR7, is a human protein encoded by the NPBWR1 gene. As implied by its name, it and related gene NPBW2 are transmembranes protein that bind Neuropeptide B (NPB) and Neuropeptide W (NPW), both proteins expressed strongly in parts of the brain that regulate stress and fear including the extended amygdala and stria terminalis. When originally discovered in 1995, these receptors had no known ligands and were called GPR7 and GPR8, but at least three groups in the early 2000s independently identified their endogenous ligands, triggering the name change in 2005.

β-Neoendorphin is an endogenous opioid peptide with a nonapeptide structure and the amino acid sequence Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro (YGGFLRKYP).

α-Neoendorphin is an endogenous opioid peptide with a decapeptide structure and the amino acid sequence Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro-Lys.

α-Endorphin (alpha-endorphin) is an endogenous opioid peptide with a length of 16 amino acids, and the amino acid sequence: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr. With the use of mass spectrometry, Nicholas Ling was able to determine the primary sequence of a-endorphin.
Neoendorphins are a group of endogenous opioid peptides derived from the proteolytic cleavage of prodynorphin. They include α-neoendorphin and β-neoendorphin. The α-neoendorphin is present in greater amounts in the brain than β-neoendorphin. Both are products of the dynorphin gene, which also expresses dynorphin A, dynorphin A-(1-8), and dynorphin B. These opioid neurotransmitters are especially active in Central Nervous System receptors, whose primary function is pain sensation. These peptides all have the consensus amino acid sequence of Try-Gly-Gly-Phe-Met (met-enkephalin) or Tyr-Gly-Gly-Phe-Leu ( leu-enkephalin). Binding of neoendorphins to opioid receptors (OPR), in the dorsal root ganglion (DRG) neurons results in the reduction of time of calcium-dependent action potential. The α-neoendorphins bind OPRD1(delta), OPRK1(kappa), and OPRM1 (mu) and β-neoendorphin bind OPRK1.
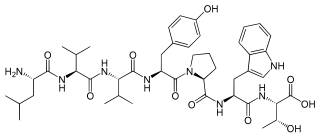
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Hemorphin-4 is an endogenous opioid peptide of the hemorphin family which possesses antinociceptive properties and is derived from the β-chain of hemoglobin in the bloodstream. It is a tetrapeptide with the amino acid sequence Tyr-Pro-Trp-Thr. Hemorphin-4 has affinities for the μ-, δ-, and κ-opioid receptors that are in the same range as the structurally related β-casomorphins, although affinity to the κ-opioid receptor is markedly higher in comparison. It acts as an agonist at these sites. Hemorphin-4 also has inhibitory effects on angiotensin-converting enzyme (ACE), and as a result, may play a role in the regulation of blood pressure. Notably, inhibition of ACE also reduces enkephalin catabolism.
Leumorphin, also known as dynorphin B1–29, is a naturally occurring endogenous opioid peptide. Derived as a proteolytic cleavage product of residues 226-254 of prodynorphin, leumorphin is a nonacosapeptide and has the sequence Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-Arg-Ser-Gln-Glu-Asp-Pro-Asn-Ala-Tyr-Ser-Gly-Glu-Leu-Phe-Asp-Ala. It can be further reduced to dynorphin B and dynorphin B-14 by pitrilysin metallopeptidase 1, an enzyme of the endopeptidase family. Leumorphin behaves as a potent and selective κ-opioid receptor agonist, similarly to other endogenous opioid peptide derivatives of prodynorphin.

Endomorphin-2 (EM-2) is an endogenous opioid peptide and one of the two endomorphins. It has the amino acid sequence Tyr-Pro-Phe-Phe-NH2. It is a high affinity, highly selective agonist of the μ-opioid receptor, and along with endomorphin-1 (EM-1), has been proposed to be the actual endogenous ligand of this receptor (that is, rather than the endorphins). Like EM-1, EM-2 produces analgesia in animals, but whereas EM-1 is more prevalent in the brain, EM-2 is more prevalent in the spinal cord. In addition, the action of EM-2 differs from that of EM-1 somewhat, because EM-2 additionally induces the release of dynorphin A and [Met]enkephalin in the spinal cord and brain by an unknown mechanism, which in turn go on to activate the κ- and δ-opioid receptors, respectively, and a portion of the analgesic effects of EM-2 is dependent on this action. Moreover, while EM-1 produces conditioned place preference, a measure of drug reward, EM-2 produces conditioned place aversion, an effect which is dynorphin A-dependent. Similarly to the case of EM-1, the gene encoding for EM-2 has not yet been identified.
CNMamide (CNMa) is a cyclic neuropeptide identified by computational analysis of Drosophila melanogaster protein sequences and named after its C-terminal ending motif. A gene encoding CNMa was found in most arthropods and comparison among the precursor sequences of several representative species revealed high conservation, particularly in the region of the predicted mature peptide. Two conserved cysteine residues enveloping four amino acids form a disulfide bond and were shown to be important for binding of the peptide to its receptor. Expression of CNMa was confirmed in the larval and adult brain of D. melanogaster but the function of the peptide has not been elucidated yet.













