
Buprenorphine is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, it is typically started when withdrawal symptoms have begun and for the first two days of treatment under direct observation of a health-care provider. In the United States, the combination formulation of buprenorphine/naloxone (Suboxone) is usually prescribed to discourage misuse by injection. Maximum pain relief is generally within an hour with effects up to 24 hours. Buprenorphine affects different types of opioid receptors in different ways. Depending on the type of receptor, it may be an agonist, partial agonist, or antagonist. In the treatment of opioid use disorder buprenorphine is an agonist/antagonist, meaning that it relieves withdrawal symptoms from other opioids and induces some euphoria, but also blocks the ability for many other opioids, including heroin, to cause an effect. Unlike full agonists like heroin or methadone, buprenorphine has a ceiling effect, such that taking more medicine will not increase the effects of the drug.

Etorphine (M99) is a semi-synthetic opioid possessing an analgesic potency approximately 1,000–3,000 times that of morphine. It was first prepared in 1960 from oripavine, which does not generally occur in opium poppy extract but rather the related plants Papaver orientale and Papaver bracteatum. It was later reproduced in 1963 by a research group at MacFarlan Smith in Gorgie, Edinburgh, led by Kenneth Bentley. It can also be produced from thebaine.
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.

Nociceptin/orphanin FQ (N/OFQ), a 17-amino acid neuropeptide, is the endogenous ligand for the nociceptin receptor, and initiates its function to act on numerous brain activities such as pain sensation and fear learning. It is derived from the prepronociceptin protein, as are a further 2 peptides, nocistatin & NocII, which inhibit the N/OFQ receptor function. Nociceptin itself acts as a potent anti-analgesic, effectively counteracting the effect of pain-relievers. The gene coding for prepronociceptin is located on Ch8p21 in humans. Nociceptin acts at the Nociceptin receptor formerly known as ORL1. Nociceptin is the first example of reverse pharmacology; the NOP receptor was discovered before the endogenous ligand which was discovered by two separate groups in 1995.
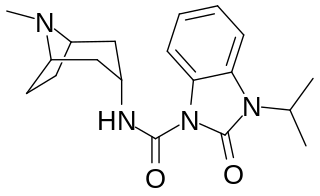
BIMU-8 is a drug which acts as a 5-HT4 receptor selective agonist. BIMU-8 was one of the first compounds of this class. The main action of BIMU-8 is to increase the rate of respiration by activating an area of the brain stem known as the pre-Botzinger complex.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.
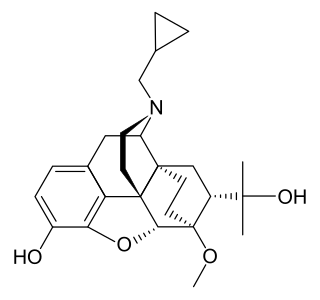
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

Nalfurafine is an antipruritic that is marketed in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis. It acts as a potent, selective, centrally-penetrant κ-opioid receptor (KOR) agonist, and is the first and currently the only selective KOR agonist to have been approved for clinical use. It has also been dubiously referred to as the "first non-narcotic opioid drug" in history.
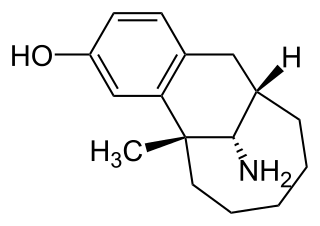
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
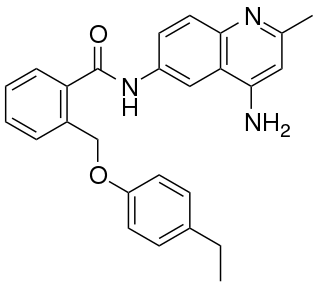
JTC-801 is an opioid analgesic drug used in scientific research.

NNC 63-0532 is a nociceptoid drug used in scientific research. It acts as a potent and selective agonist for the nociceptin receptor, also known as the ORL-1 receptor.

Spiradoline (U-62066) is a drug which acts as a highly selective κ-opioid agonist. It has analgesic, diuretic, and antitussive effects, and produces subjective effects in animals similar to those of ketazocine and alazocine. The main effect in humans is sedation, along with analgesic and diuretic effects, but significant side effects such as dysphoria and hallucinations have stopped it from being used clinically.
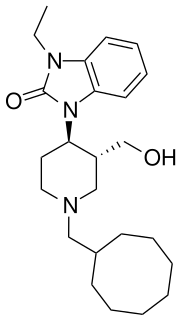
J-113,397 is an opioid drug which was the first compound found to be a highly selective antagonist for the nociceptin receptor, also known as the ORL-1 receptor. It is several hundred times selective for the ORL-1 receptor over other opioid receptors, and its effects in animals include preventing the development of tolerance to morphine, the prevention of hyperalgesia induced by intracerebroventricular administration of nociceptin, as well as the stimulation of dopamine release in the striatum, which increases the rewarding effects of cocaine, but may have clinical application in the treatment of Parkinson's disease.

SB-612,111 is an opioid receptor ligand which is a potent and selective antagonist for the nociceptin receptor (ORL-1), several times more potent than the older drug J-113,397. It does not have analgesic effects in its own right, but prevents the development of hyperalgesia, and also shows antidepressant effects in animal studies.

Ro64-6198 is a nociceptoid drug used in scientific research. It acts as a potent and selective agonist for the nociceptin receptor, also known as the ORL-1 receptor, with over 100x selectivity over other opioid receptors. It produces anxiolytic effects in animal studies equivalent to those of benzodiazepine drugs, but has no anticonvulsant effects and does not produce any overt effects on behaviour. However it does impair short-term memory, and counteracts stress-induced anorexia. It also has antitussive effects, and reduces the rewarding and analgesic effects of morphine, although it did not prevent the development of dependence. It has been shown to reduce alcohol self-administration in animals and suppressed relapses in animal models of alcoholism, and ORL-1 agonists may have application in the treatment of alcoholism.
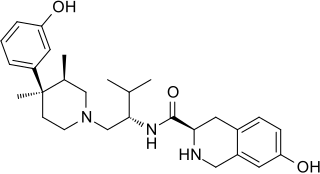
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].
Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor. Osemozotan has antidepressant, anxiolytic, antiobsessional, serenic, and analgesic effects in animal studies, and is used to investigate the role of 5-HT1A receptors in modulating the release of dopamine and serotonin in the brain, and their involvement in addiction to abused stimulants such as cocaine and methamphetamine.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

Olivier Civelli is a molecular biologist, a researcher in the field of neuropharmacology and an educator. He is the Eric L. and Lila D. Nelson Professor of Neuropharmacology at University of California, Irvine. He is also a Professor in the Department of Developmental and Cell Biology at University of California, Irvine. He is most known for his work in advancing understanding of neurotransmission and his impact on drug discovery.

SR-16435 is a drug which acts as a potent partial agonist at both the μ-opioid receptor and nociceptin receptor. In animal studies it was found to be a potent analgesic, with results suggestive of reduced development of tolerance and increased activity against neuropathic pain compared to classic μ-selective agonists.


















