
Fentanyl is a potent synthetic piperidine opioid primarily used as an analgesic. It is 20 to 40 times more potent than heroin and 100 times more potent than morphine; its primary clinical utility is in pain management for cancer patients and those recovering from painful surgeries. Fentanyl is also used as a sedative. Depending on the method of delivery, fentanyl can be very fast acting and ingesting a relatively small quantity can cause overdose. Fentanyl works by activating μ-opioid receptors. Fentanyl is sold under the brand names Actiq, Duragesic and Sublimaze, among others.

α-Methylfentanyl an opioid analgesic that is an analog of fentanyl. It is sometimes sold as "China White".
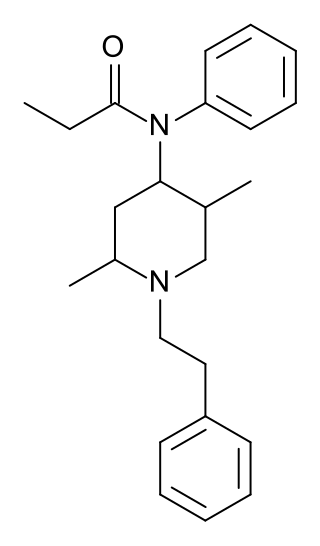
Phenaridine (2,5-dimethylfentanyl) is an opioid analgesic that is an analogue of fentanyl. It was developed in 1972, and is used for surgical anasthesia.

Ocfentanil is a potent synthetic opioid structurally related to fentanyl that was developed in the early 1990s as one of a series of potent naloxone-reversible opioids in an attempt to obtain an opioid that had better therapeutic indices in terms of cardiovascular effects and respiratory depression as compared to fentanyl. Ocfentanil was never developed for medical use despite reasonable results in human clinical trials, but subsequently started to be sold as a designer drug starting in around 2013.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.
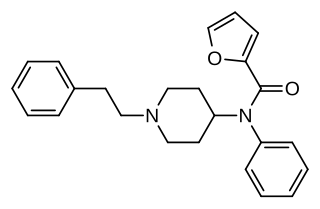
Furanylfentanyl (Fu-F) is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. It has an ED50 value of 0.02 mg/kg in mice. This makes it approximately one fifth as potent as fentanyl.

3-Methylbutyrfentanyl (3-MBF) is an opioid analgesic that is an analog of butyrfentanyl.

4-Fluorobutyrylfentanyl (also known as 4-FBF and p-FBF or para-fluorobutyrylfentanyl) is an opioid analgesic that is an analog of butyrfentanyl and has been sold online as a designer drug. It is closely related to 4-fluorofentanyl, which has an EC50 value of 4.2 nM for the human μ-opioid receptor.

4-Methoxybutyrfentanyl is an opioid analgesic that is an analog of butyrfentanyl and has been sold online as a designer drug.
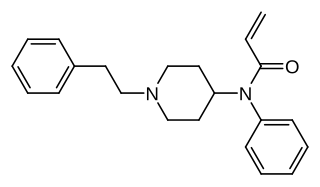
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.

Methoxyacetylfentanyl, commonly known as MAF is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug.
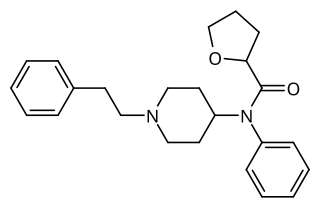
Tetrahydrofuranylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug, first appearing in Europe in late 2016.

Cyclopentylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug, mainly in Sweden and other Scandinavian countries.

4-Fluoroisobutyrylfentanyl (also known as 4-FIBF and p-FIBF) is an opioid analgesic that is an analog of butyrfentanyl and structural isomer of 4-Fluorobutyrfentanyl and has been sold online as a designer drug. It is closely related to 4-fluorofentanyl, which has an EC50 value of 4.2 nM for the human μ-opioid receptor. 4-fluoroisobutyrylfentanyl is a highly selective μ-opioid receptor agonist whose analgesic potency is almost ten times of that reported for morphine.
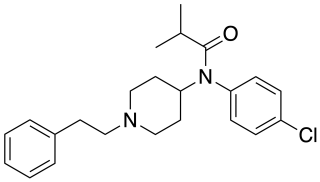
4-Chloroisobutyrylfentanyl is an opioid analgesic that is an analog of fentanyl, and has been sold online as a designer drug.

Butyrnorfentanyl or butyrylnorfentanyl is an inactive synthetic opioid analgesic drug precursor. It is an analog of fentanyl.

Despropionyl-p-fluorofentanyl is an inactive synthetic opioid analgesic drug precursor to 4-fluorofentanyl. It is an analog of fentanyl.

Furanylnorfentanyl is an inactive synthetic opioid analgesic drug precursor. It is an analog of fentanyl.

Norfentanyl is an inactive synthetic opioid analgesic drug precursor. It is an analog and metabolite of fentanyl with the removal of the phenethyl moiety from fentanyl chemical structure.

Remifentanilic acid is a metabolite of the potent short-acting synthetic opioid analgesic drug remifentanil. It is an analog of fentanyl and remifentanil, but is not active as an opioid in its own right.





















