
Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the twelfth letter of the Greek alphabet. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.

The ∆-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the ∆-opioid receptor is largely expressed vary from species model to species model. In humans, the ∆-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.

TRIMU-5 is a selective agonist of the μ2-opioid receptor and antagonist of the μ1-opioid receptor. It produces analgesia in animals that differs from that of conventional μ-opioid receptor agonists but that can still be blocked by μ-opioid receptor antagonists. TRIMU-5 can also block the analgesic effects of μ-opioid receptor agonists like morphine. In addition to analgesia, TRIMU-5 inhibits gastrointestinal transit, a known effect of μ2-opioid receptor activation.
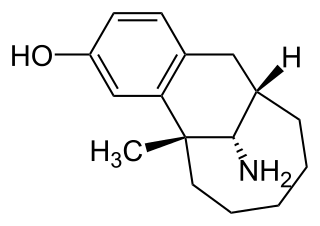
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
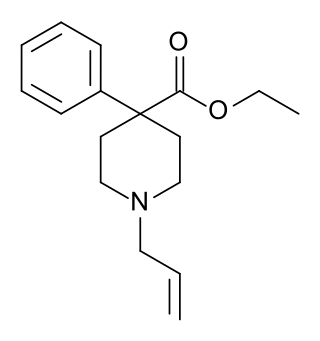
Allylnorpethidine (WIN-7681) is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).

Levallorphan, also known as levallorphan tartrate (USAN), is an opioid modulator of the morphinan family used as an opioid analgesic and opioid antagonist/antidote. It acts as an antagonist of the μ-opioid receptor (MOR) and as an agonist of the κ-opioid receptor (KOR), and as a result, blocks the effects of stronger agents with greater intrinsic activity such as morphine whilst simultaneously producing analgesia.

Azidomorphine is an opiate analogue that is a derivative of morphine, where the 7,8 double bond has been saturated and the 6-hydroxy group has been replaced by an azide group.

DPI-3290 was discovered by scientists at Burroughs Wellcome and licensed to Delta Pharmaceutical and is a drug that is used in scientific research. It is a potent analgesic drug, which produces little respiratory depression.

RWJ-394674 is a drug that is used in scientific research. It is a potent, orally active analgesic drug that produces little respiratory depression. RWJ-394674 itself is a potent and selective agonist for δ-opioid receptors, with a Ki of 0.24 nM at δ and 72 nM at μ. However once inside the body, RWJ-394674 is dealkylated to its monodesethyl metabolite RWJ-413216, which is a potent agonist at the μ-opioid receptor and has less affinity for δ. The effect of RWJ-394674 when administered in vivo thus produces potent agonist effects at both μ and δ receptors through the combined actions of the parent drug and its active metabolite, with the δ-agonist effects counteracting the respiratory depression from the μ-opioid effects, and the only prominent side-effect being sedation.

SC-17599 is a steroid derivative drug discovered in 1968 which acts as a selective μ-opioid receptor agonist, with little or no affinity for the δ-opioid or κ-opioid receptors. It is an active analgesic in vivo, more potent than codeine or pethidine but slightly less potent than morphine, and produces similar effects to morphine in animals but with less sedation

Oxymorphazone is an opioid analgesic drug related to oxymorphone. Oxymorphazone is a potent and long acting μ-opioid agonist which binds irreversibly to the receptor, forming a covalent bond which prevents it from detaching once bound. This gives it an unusual pharmacological profile, and while oxymorphazone is only around half the potency of oxymorphone, with higher doses the analgesic effect becomes extremely long lasting, with a duration of up to 48 hours. However, tolerance to analgesia develops rapidly with repeated doses, as chronically activated opioid receptors are rapidly internalised by β-arrestins, similar to the results of non-covalent binding by repeated doses of agonists with extremely high binding affinity such as lofentanil.

An opiate, in classical pharmacology, is a substance derived from opium. In more modern usage, the term opioid is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

IBNtxA, or 3-iodobenzoyl naltrexamine, is an atypical opioid analgesic drug derived from naltrexone. In animal studies it produces potent analgesic effects that are blocked by levallorphan and so appear to be μ-opioid mediated, but it fails to produce constipation or respiratory depression, and is neither rewarding or aversive in conditioned place preference protocols. These unusual properties are thought to result from agonist action at a splice variant or heterodimer of the μ-opioid receptor, rather than at the classical full length form targeted by conventional opioid drugs.
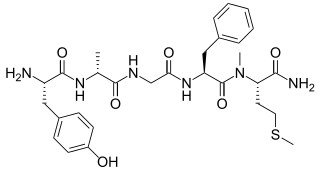
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

PZM21 is an experimental opioid analgesic drug that is being researched for the treatment of pain. It is claimed to be a functionally selective μ-opioid receptor agonist which produces μ-opioid receptor mediated G protein signaling, with potency and efficacy similar to morphine, but with less β-arrestin 2 recruitment. However, recent reports highlight that this might be due to its low intrinsic efficacy, rather than functional selectivity or 'G protein bias' as initially reported. In tests on mice, PZM21 was slightly less potent than morphine or TRV130 as an analgesic, but also had significantly reduced adverse effects, with less constipation than morphine, and very little respiratory depression, even at high doses. This research was described as a compelling example of how modern high-throughput screening techniques can be used to discover new chemotypes with specific activity profiles, even at targets such as the μ-opioid receptor which have already been thoroughly investigated. More recent research has suggested however that at higher doses, PZM21 is capable of producing classic opioid side effects such as respiratory depression and development of tolerance and may have only limited functional selectivity.
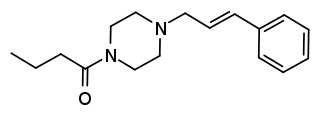
Bucinnazine is an opioid analgesic drug that was widely used in China to treat pain in cancer patients as of 1986. It is one of the most potent compounds among a series of piperazine-amides first synthesized and reported in Japan in the 1970s. Bucinnazine has analgesic potency comparable to that of morphine but with a relatively higher therapeutic index.

HS665 is a drug which acts as a potent and selective κ-opioid receptor agonist, and has analgesic effects in animal studies. HS665 is not an agonist for the mu receptor, leading to less potential for abuse.




















