
Hematite, also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of Fe
2O
3. It has the same crystal structure as corundum (Al
2O
3) and ilmenite (FeTiO
3). With this it forms a complete solid solution at temperatures above 950 °C (1,740 °F).

Pottery is the process and the products of forming vessels and other objects with clay and other raw materials, which are fired at high temperatures to give them a hard and durable form. The place where such wares are made by a potter is also called a pottery. The definition of pottery, used by the ASTM International, is "all fired ceramic wares that contain clay when formed, except technical, structural, and refractory products". End applications include tableware, decorative ware, sanitaryware, and in technology and industry such as electrical insulators and laboratory ware. In art history and archaeology, especially of ancient and prehistoric periods, pottery often means vessels only, and sculpted figurines of the same material are called terracottas.

Raku ware is a type of Japanese pottery traditionally used in Japanese tea ceremonies, most often in the form of chawan tea bowls. It is traditionally characterised by being hand-shaped rather than thrown, fairly porous vessels, which result from low firing temperatures, lead glazes and the removal of pieces from the kiln while still glowing hot. In the traditional Japanese process, the fired raku piece is removed from the hot kiln and is allowed to cool in the open air.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide.

Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.

Earthenware is glazed or unglazed nonvitreous pottery that has normally been fired below 1,200 °C (2,190 °F). Basic earthenware, often called terracotta, absorbs liquids such as water. However, earthenware can be made impervious to liquids by coating it with a ceramic glaze, and is used for the great majority of modern domestic earthenware. The main other important types of pottery are porcelain, bone china, and stoneware, all fired at high enough temperatures to vitrify. End applications include tableware and decorative ware such as figurines.

Wüstite (FeO) is a mineral form of iron(II) oxide found with meteorites and native iron. It has a grey colour with a greenish tint in reflected light. Wüstite crystallizes in the isometric-hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a specific gravity of 5.88. Wüstite is a typical example of a non-stoichiometric compound.

Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same formula as hematite, but the same spinel ferrite structure as magnetite (Fe3O4) and is also ferrimagnetic. It is sometimes spelled as "maghaemite".

Pottery, due to its relative durability, comprises a large part of the archaeological record of ancient Greece, and since there is so much of it, it has exerted a disproportionately large influence on our understanding of Greek society. The shards of pots discarded or buried in the 1st millennium BC are still the best guide available to understand the customary life and mind of the ancient Greeks. There were several vessels produced locally for everyday and kitchen use, yet finer pottery from regions such as Attica was imported by other civilizations throughout the Mediterranean, such as the Etruscans in Italy. There were a multitude of specific regional varieties, such as the South Italian ancient Greek pottery.

Iron(II,III) oxide, or black iron oxide, is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occurs naturally as the mineral hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment (see: Mars Black). For this purpose, it is synthesized rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.

Metal clay is a crafting medium consisting of very small particles of metal such as silver, gold, bronze, or copper mixed with an organic binder and water for use in making jewelry, beads and small sculptures. Originating in Japan in 1990, metal clay can be shaped just like any soft clay, by hand or using molds. After drying, the clay can be fired in a variety of ways such as in a kiln, with a handheld gas torch, or on a gas stove, depending on the type of clay and the metal in it. The binder burns away, leaving the pure sintered metal. Shrinkage of between 8% and 30% occurs. Alloys such as bronze, sterling silver, and steel also are available.

Tin-glazing is the process of giving tin-glazed pottery items a ceramic glaze that is white, glossy and opaque, which is normally applied to red or buff earthenware. Tin-glaze is plain lead glaze with a small amount of tin oxide added. The opacity and whiteness of tin glaze encourage its frequent decoration. Historically this has mostly been done before the single firing, when the colours blend into the glaze, but since the 17th century also using overglaze enamels, with a light second firing, allowing a wider range of colours. Majolica, maiolica, delftware and faience are among the terms used for common types of tin-glazed pottery.
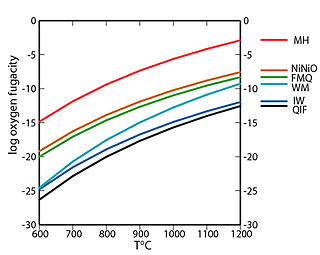
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Bucchero is a class of ceramics produced in central Italy by the region's pre-Roman Etruscan population. This Italian word is derived from the Latin poculum, a drinking-vessel, perhaps through the Spanish búcaro, or the Portuguese púcaro.
This is a list of pottery and ceramic terms.

The surface color of the planet Mars appears reddish from a distance because of rusty atmospheric dust. From close up, it looks more of a butterscotch, and other common surface colors include golden, brown, tan, and greenish, depending on minerals.

Mill scale, often shortened to just scale, is the flaky surface of hot rolled steel, consisting of the mixed iron oxides iron(II) oxide (FeO), iron(III) oxide, and iron(II,III) oxide.

Iron oxide nanoparticles are iron oxide particles with diameters between about 1 and 100 nanometers. The two main forms are composed of magnetite and its oxidized form maghemite. They have attracted extensive interest due to their superparamagnetic properties and their potential applications in many fields including molecular imaging.

The Schikorr reaction formally describes the conversion of the iron(II) hydroxide (Fe(OH)2) into iron(II,III) oxide (Fe3O4). This transformation reaction was first studied by Gerhard Schikorr. The global reaction follows:

Black-topped pottery is a specialized type of Ancient Egyptian pottery that was found in Nubian archaeological sites, including Elephantine, an island on the Nile River, Nabta Playa in the Nubian Desert, and Kerma in present-day Sudan. This type of artifact dates predominantly to the Predynastic Period, but “a handful of examples made in the Early Dynastic Period are known to exist.” These vessels were used “exclusively for ritual and funerary purposes” and were discovered in ancient cemeteries and settlements. The majority of these pots are variations of the Egyptian hes-jar form and feature red bodies with black tops and interiors. The red color is derived from the natural iron that occurs within Nile silts which oxidizes upon firing, and the black top and interior is a product of reduction firing and carbon smudging.























