Related Research Articles

A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include T cells, B cells, and Innate lymphoid cells (ILCs), of which natural killer cells are an important subtype. They are the main type of cell found in lymph, which prompted the name "lymphocyte". Lymphocytes make up between 18% and 42% of circulating white blood cells.

A biopsy is a medical test commonly performed by a surgeon, interventional radiologist, or an interventional cardiologist. The process involves extraction of sample cells or tissues for examination to determine the presence or extent of a disease. The tissue is then fixed, dehydrated, embedded, sectioned, stained and mounted before it is generally examined under a microscope by a pathologist; it may also be analyzed chemically. When an entire lump or suspicious area is removed, the procedure is called an excisional biopsy. An incisional biopsy or core biopsy samples a portion of the abnormal tissue without attempting to remove the entire lesion or tumor. When a sample of tissue or fluid is removed with a needle in such a way that cells are removed without preserving the histological architecture of the tissue cells, the procedure is called a needle aspiration biopsy. Biopsies are most commonly performed for insight into possible cancerous or inflammatory conditions.

Flow cytometry (FC) is a technique used to detect and measure physical and chemical characteristics of a population of cells or particles.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.
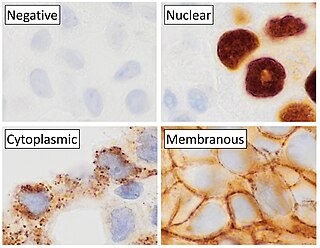
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue. Albert Coons conceptualized and first implemented the procedure in 1941.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells. Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells. Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.

GD2 is a disialoganglioside expressed on tumors of neuroectodermal origin, including human neuroblastoma and melanoma, with highly restricted expression on normal tissues, principally to the cerebellum and peripheral nerves in humans.

FOXP3, also known as scurfin, is a protein involved in immune system responses. A member of the FOX protein family, FOXP3 appears to function as a master regulator of the regulatory pathway in the development and function of regulatory T cells. Regulatory T cells generally turn the immune response down. In cancer, an excess of regulatory T cell activity can prevent the immune system from destroying cancer cells. In autoimmune disease, a deficiency of regulatory T cell activity can allow other autoimmune cells to attack the body's own tissues.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.

Exosomes are membrane-bound extracellular vesicles (EVs) that are produced in the endosomal compartment of most eukaryotic cells. In multicellular organisms, exosomes and other EVs are found in biological fluids including saliva, blood, urine and cerebrospinal fluid. EVs have specialized functions in physiological processes, from coagulation and waste management to intercellular communication.
Flow-FISH is a cytogenetic technique to quantify the copy number of RNA or specific repetitive elements in genomic DNA of whole cell populations via the combination of flow cytometry with cytogenetic fluorescent in situ hybridization staining protocols.

Mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells or medicinal signaling cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes and adipocytes.
MHC multimers are oligomeric forms of MHC molecules, designed to identify and isolate T-cells with high affinity to specific antigens amid a large group of unrelated T-cells. Multimers generally range in size from dimers to octamers; however, some companies use even higher quantities of MHC per multimer. Multimers may be used to display class 1 MHC, class 2 MHC, or nonclassical molecules from species such as monkeys, mice, and humans.

Mass cytometry is a mass spectrometry technique based on inductively coupled plasma mass spectrometry and time of flight mass spectrometry used for the determination of the properties of cells (cytometry). In this approach, antibodies are conjugated with isotopically pure elements, and these antibodies are used to label cellular proteins. Cells are nebulized and sent through an argon plasma, which ionizes the metal-conjugated antibodies. The metal signals are then analyzed by a time-of-flight mass spectrometer. The approach overcomes limitations of spectral overlap in flow cytometry by utilizing discrete isotopes as a reporter system instead of traditional fluorophores which have broad emission spectra.

In cell biology, single-cell variability occurs when individual cells in an otherwise similar population differ in shape, size, position in the cell cycle, or molecular-level characteristics. Such differences can be detected using modern single-cell analysis techniques. Investigation of variability within a population of cells contributes to understanding of developmental and pathological processes,
A T memory stem cell (TSCM) is a type of long-lived memory T cell with the ability to reconstitute the full diversity of memory and effector T cell subpopulations as well as to maintain their own pool through self-renewal. TSCM represent an intermediate subset between naïve (Tn) and central memory (Tcm) T cells, expressing both naïve T cells markers, such as CD45RA+, CD45RO-, high levels of CD27, CD28, IL-7Rα (CD127), CD62L, and C-C chemokine receptor 7 (CCR7), as well as markers of memory T cells, such as CD95, CD122 (IL-2Rβ), CXCR3, LFA-1. These cells represent a small fraction of circulating T cells, approximately 2-3%. Like naïve T cells, TSCM cells are found more abundantly in lymph nodes than in the spleen or bone marrow; but in contrast to naïve T cells, TSCM cells are clonally expanded. Similarly to memory T cells, TSCM are able to rapidly proliferate and secrete pro-inflammatory cytokines in response to antigen re-exposure, but show higher proliferation potential compared with Tcm cells; their homeostatic turnover is also dependent on IL-7 and IL-15.
References
- 1 2 "Image Cytometry Technology and Tissue Analysis". News-Medical.net. 2018-09-04. Retrieved 2022-07-07.
- 1 2 Cualing, Hernani D.; Zhong, Eric; Moscinski, Lynn (2006). ""Virtual flow cytometry" of immunostained lymphocytes on microscopic tissue slides:iHCFlow™ tissue cytometry". Cytometry Part B. 72B (1): 63–76. doi: 10.1002/cyto.b.20148 . ISSN 1552-4949. PMID 17133379. S2CID 36237785.
- ↑ "AI in Tissue Cytometry".
- 1 2 "Home". tissuegnostics.com. Retrieved 2021-08-04.
- ↑ Ferkowicz, Michael J.; Winfree, Seth; Sabo, Angela R.; Kamocka, Malgorzata M.; Khochare, Suraj; Barwinska, Daria; Eadon, Michael T.; Cheng, Ying-Hua; Phillips, Carrie L.; Sutton, Timothy A.; Kelly, Katherine J. (2021). "Large-scale, three-dimensional tissue cytometry of the human kidney: a complete and accessible pipeline". Laboratory Investigation. 101 (5): 661–676. doi:10.1038/s41374-020-00518-w. ISSN 1530-0307. PMC 8363780 . PMID 33408350.
- 1 2 Duraiyan, Jeyapradha; Govindarajan, Rajeshwar; Kaliyappan, Karunakaran; Palanisamy, Murugesan (August 2012). "Applications of immunohistochemistry". Journal of Pharmacy & Bioallied Sciences. 4 (Suppl 2): S307–S309. doi: 10.4103/0975-7406.100281 . ISSN 0976-4879. PMC 3467869 . PMID 23066277.
- ↑ Imaging Modalities for Biological and Preclinical Research: A Compendium Volume One. IOP Publishing. 2021. pp. I.2.h-1-I.2.h-10.
- ↑ McKinnon, Katherine M. (2018-02-21). "Flow Cytometry: An Overview". Current Protocols in Immunology. 120: 5.1.1–5.1.11. doi:10.1002/cpim.40. ISSN 1934-3671. PMC 5939936 . PMID 29512141.
- ↑ Sanderson, Michael J.; Smith, Ian; Parker, Ian; Bootman, Martin D. (2014-10-01). "Fluorescence Microscopy". Cold Spring Harbor Protocols. 2014 (10): pdb.top071795. doi:10.1101/pdb.top071795. ISSN 1940-3402. PMC 4711767 . PMID 25275114.
- ↑ "Tissue Cytometry | Flow Cytometry | What's the Difference?". tissuegnostics.com. Retrieved 2022-07-04.
- ↑ El-Achkar, Tarek M.; Winfree, Seth; Talukder, Niloy; Barwinska, Daria; Ferkowicz, Michael J.; Al Hasan, Mohammad (2022). "Tissue Cytometry With Machine Learning in Kidney: From Small Specimens to Big Data". Frontiers in Physiology. 13: 832457. doi: 10.3389/fphys.2022.832457 . ISSN 1664-042X. PMC 8931540 . PMID 35309077.
- ↑ USPTO.report. "Method and system for analyzing cells Patent Application". USPTO.report. Retrieved 2022-07-07.
- ↑ Cualing, Hernani D.; Zhong, Eric; Moscinski, Lynn (2007-01-15). ""Virtual flow cytometry" of immunostained lymphocytes on microscopic tissue slides: iHCFlow tissue cytometry". Cytometry Part B. 72 (1): 63–76. doi: 10.1002/cyto.b.20148 . ISSN 1552-4949. PMID 17133379. S2CID 36237785.
- ↑ Gillette, Paul C.; Lando, Jerome B.; Koenig, Jack L. (1983-04-01). "Factor analysis for separation of pure component spectra from mixture spectra". Analytical Chemistry. 55 (4): 630–633. doi:10.1021/ac00255a011. ISSN 0003-2700.
- ↑ Zhou, R.; Parker, D. L.; Hammond, E. H. (1992). "Quantitative peroxidase-antiperoxidase complex-substrate mass determination in tissue sections by a dual wavelength method". Analytical and Quantitative Cytology and Histology. 14 (2): 73–80. PMID 1590900.
- 1 2 3 Mungenast, Felicitas; Fernando, Achala; Nica, Robert; Boghiu, Bogdan; Lungu, Bianca; Batra, Jyotsna; Ecker, Rupert C. (2021-04-07). "Next-Generation Digital Histopathology of the Tumor Microenvironment". Genes. 12 (4): 538. doi: 10.3390/genes12040538 . ISSN 2073-4425. PMC 8068063 . PMID 33917241.
- ↑ Gerner, Michael Y.; Kastenmuller, Wolfgang; Ifrim, Ina; Kabat, Juraj; Germain, Ronald N. (2012-08-24). "Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes". Immunity. 37 (2): 364–376. doi:10.1016/j.immuni.2012.07.011. ISSN 1097-4180. PMC 3514885 . PMID 22863836.
- ↑ Allard-Chamard, Hugues; Alsufyani, Faisal; Kaneko, Naoki; Xing, Kelly; Perugino, Cory; Mahajan, Vinay S.; Wheat, Joseph L.; Deepe, George S.; Loyd, James; Pillai, Shiv (2021-02-01). "CD4+CTLs in Fibrosing Mediastinitis Linked to Histoplasma capsulatum". The Journal of Immunology. 206 (3): 524–530. doi:10.4049/jimmunol.2000433. ISSN 0022-1767. PMC 7978153 . PMID 33328214.
- ↑ Munemura, Ryusuke; Maehara, Takashi; Murakami, Yuka; Koga, Risako; Aoyagi, Ryuichi; Kaneko, Naoki; Doi, Atsushi; Perugino, Cory A.; Della-Torre, Emanuel; Saeki, Takako; Sato, Yasuharu; Yamamoto, Hidetaka; Kiyoshima, Tamotsu; Stone, John H.; Pillai, Shiv (August 2022). "Distinct disease-specific Tfh cell populations in 2 different fibrotic diseases: IgG4-related disease and Kimura disease". The Journal of Allergy and Clinical Immunology. 150 (2): 440–455.e17. doi:10.1016/j.jaci.2022.03.034. ISSN 1097-6825. PMC 10369367 . PMID 35568079.
- ↑ Ding, Dah-Ching; Shyu, Woei-Cherng; Lin, Shinn-Zong (2011). "Mesenchymal stem cells". Cell Transplantation. 20 (1): 5–14. doi:10.3727/096368910X. ISSN 1555-3892. PMID 21396235. S2CID 7868466.
- ↑ Baer, Patrick C. (2014-07-26). "Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro". World Journal of Stem Cells. 6 (3): 256–265. doi: 10.4252/wjsc.v6.i3.256 . ISSN 1948-0210. PMC 4131268 . PMID 25126376.
- ↑ Wong, Tzyy Yue; Chang, Chiung-Hsin; Yu, Chen-Hsiang; Huang, Lynn L. H. (June 2017). "Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time". Aging Cell. 16 (3): 451–460. doi:10.1111/acel.12567. ISSN 1474-9726. PMC 5418204 . PMID 28474484.
- ↑ Pillat, Micheli Mainardi; Oliveira-Giacomelli, Ágatha; das Neves Oliveira, Mona; Andrejew, Roberta; Turrini, Natalia; Baranova, Juliana; Lah Turnšek, Tamara; Ulrich, Henning (February 2021). "Mesenchymal stem cell-glioblastoma interactions mediated via kinin receptors unveiled by cytometry". Cytometry. Part A. 99 (2): 152–163. doi: 10.1002/cyto.a.24299 . ISSN 1552-4930. PMID 33438373. S2CID 231594242.
- ↑ Campos, Henrique C.; Ribeiro, Deidiane Elisa; Hashiguchi, Debora; Hukuda, Deborah Y.; Gimenes, Christiane; Romariz, Simone A. A.; Ye, Qing; Tang, Yong; Ulrich, Henning; Longo, Beatriz Monteiro (February 2022). "Distinct Effects of the Hippocampal Transplantation of Neural and Mesenchymal Stem Cells in a Transgenic Model of Alzheimer's Disease". Stem Cell Reviews and Reports. 18 (2): 781–791. doi:10.1007/s12015-021-10321-9. ISSN 2629-3277. PMID 34997526. S2CID 255446340.
- ↑ Kaneko, Naoki; Boucau, Julie; Kuo, Hsiao-Hsuan; Perugino, Cory; Mahajan, Vinay S.; Farmer, Jocelyn R.; Liu, Hang; Diefenbach, Thomas J.; Piechocka-Trocha, Alicja; Lefteri, Kristina; Waring, Michael T.; Premo, Katherine R.; Walker, Bruce D.; Li, Jonathan Z.; Gaiha, Gaurav (April 2022). "Temporal changes in T cell subsets and expansion of cytotoxic CD4+ T cells in the lungs in severe COVID-19". Clinical Immunology (Orlando, Fla.). 237: 108991. doi:10.1016/j.clim.2022.108991. ISSN 1521-7035. PMC 8961941 . PMID 35364330.
- ↑ Kaneko, Naoki; Kuo, Hsiao-Hsuan; Boucau, Julie; Farmer, Jocelyn R.; Allard-Chamard, Hugues; Mahajan, Vinay S.; Piechocka-Trocha, Alicja; Lefteri, Kristina; Osborn, Matthew; Bals, Julia; Bartsch, Yannic C.; Bonheur, Nathalie; Caradonna, Timothy M.; Chevalier, Josh; Chowdhury, Fatema (2020-10-01). "Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19". Cell. 183 (1): 143–157.e13. doi:10.1016/j.cell.2020.08.025. ISSN 1097-4172. PMC 7437499 . PMID 32877699.
- 1 2 Semeano, Ana T.; Tofoli, Fabiano A.; Corrêa-Velloso, Juliana C.; de Jesus Santos, Ana P.; Oliveira-Giacomelli, Ágatha; Cardoso, Rafaela R.; Pessoa, Mateus A.; da Rocha, Edroaldo Lummertz; Ribeiro, Gustavo; Ferrari, Merari F. R.; Pereira, Lygia V.; Teng, Yang D.; Petri, Denise F. S.; Ulrich, Henning (April 2022). "Effects of Magnetite Nanoparticles and Static Magnetic Field on Neural Differentiation of Pluripotent Stem Cells". Stem Cell Reviews and Reports. 18 (4): 1337–1354. doi:10.1007/s12015-022-10332-0. ISSN 2629-3277. PMID 35325357. S2CID 247678247.
- 1 2 Yi, Shanyong; Chen, Ke; Zhang, Lihua; Shi, Weibo; Zhang, Yaxing; Niu, Shiba; Jia, Miaomiao; Cong, Bin; Li, Yingmin (2019). "Endoplasmic Reticulum Stress Is Involved in Stress-Induced Hypothalamic Neuronal Injury in Rats via the PERK-ATF4-CHOP and IRE1-ASK1-JNK Pathways". Frontiers in Cellular Neuroscience. 13: 190. doi: 10.3389/fncel.2019.00190 . ISSN 1662-5102. PMC 6509942 . PMID 31130849.
- ↑ Sze, Chun-I.; Lin, Yung-Chieh; Lin, Yuh-Jyh; Hsieh, Ting-Hui; Kuo, Yu Min; Lin, Chyi-Her (2013). "The role of glucocorticoid receptors in dexamethasone-induced apoptosis of neuroprogenitor cells in the hippocampus of rat pups". Mediators of Inflammation. 2013: 628094. doi: 10.1155/2013/628094 . ISSN 1466-1861. PMC 3557631 . PMID 23401645.
- ↑ Wang, Liang-Chao; Huang, Chih-Yuan; Wang, Hao-Kuang; Wu, Ming-Hsiu; Tsai, Kuen-Jer (2012-05-01). "Magnesium sulfate and nimesulide have synergistic effects on rescuing brain damage after transient focal ischemia". Journal of Neurotrauma. 29 (7): 1518–1529. doi:10.1089/neu.2011.2030. ISSN 1557-9042. PMC 3335109 . PMID 22332641.