
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

Hydroxylamine is an inorganic compound with the chemical formula NH2OH. The compound is in a form of a white hygroscopic crystals. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. The oxidation of NH3 to hydroxylamine is a step in biological nitrification.

In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.

Potassium tetraiodomercurate(II) is an inorganic compound with the chemical formula K2[HgI4]. It consists of potassium cations and tetraiodomercurate(II) anions. It is the active agent in Nessler's reagent, used for detection of ammonia.
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests a salt with the composition [NH+
4][OH−
], it is actually impossible to isolate samples of NH4OH. The ions NH+
4 and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions.
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was first developed by Johannes Nicolaus Brønsted and Thomas Martin Lowry independently in 1923. The basic concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory generalises the Arrhenius theory.
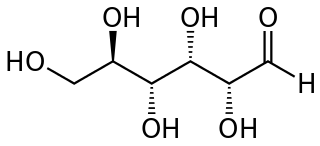
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.

Silver chloride is an inorganic chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
The sodium fusion test, or Lassaigne's test, is used in elemental analysis for the qualitative determination of the presence of foreign elements, namely halogens, nitrogen, and sulfur, in an organic compound. It was developed by J. L. Lassaigne.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.
A salt metathesis reaction is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme:

Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
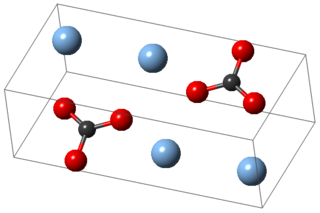
Silver carbonate is the chemical compound with the formula Ag2CO3. This salt is yellow but typical samples are grayish due to the presence of elemental silver. It is poorly soluble in water, like most transition metal carbonates.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
























