
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, hydrocarbons are divided into two classes: aromatic compounds and aliphatic compounds. Aliphatic compounds can be saturated like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic.

In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.
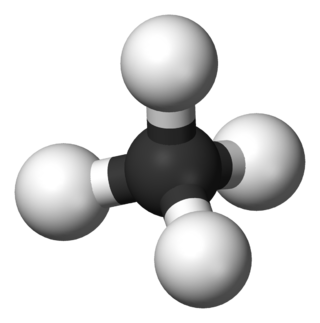
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition. These isomers are flammable liquids. Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene. Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.

RP-1 (alternatively, Rocket Propellant-1 or Refined Petroleum-1) is a highly refined form of kerosene outwardly similar to jet fuel, used as rocket fuel. RP-1 provides a lower specific impulse than liquid hydrogen (H2), but is cheaper, is stable at room temperature, and presents a lower explosion hazard. RP-1 is far denser than H2, giving it a higher energy density (though its specific energy is lower). RP-1 also has a fraction of the toxicity and carcinogenic hazards of hydrazine, another room-temperature liquid fuel.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
In organic chemistry, a cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms and either one or more double bonds, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the cis isomers, and the term cis tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond; however, this is not feasible in smaller molecules due to the increase of strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of CH2 substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In larger rings, cis–trans isomerism of the double bond may occur. This stability pattern forms part of the origin of Bredt's rule, the observation that alkenes do not form at the bridgehead of many types of bridged ring systems because the alkene would necessarily be trans in one of the rings.
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.

In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, single chemical bond connecting two sp3-hybridised atoms, and it is normally a conformational energy maximum. This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation.
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2:
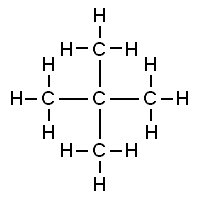
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Diamond and graphite are a familiar example; they are isomers of carbon. Isomerism refers to the existence or possibility of isomers.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.
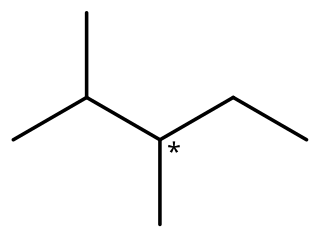
2,3-Dimethylpentane is an organic compound of carbon and hydrogen with formula C
7H
16, more precisely CH
3–CH(CH
3)–CH(CH
3)–CH
2–CH
3: a molecule of pentane with methyl groups –CH
3 replacing hydrogen atoms on carbon atoms 2 and 3. It is an alkane, a fully saturated hydrocarbon; specifically, one of the isomers of heptane.

















