Related Research Articles

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.

The Cochrane Collaboration is a British international charitable organisation formed to synthesise medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.
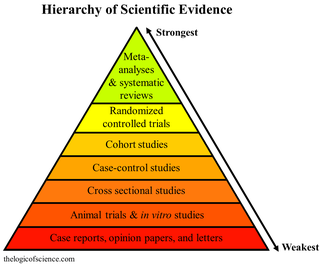
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence. Some case reports also contain a literature review of other reported cases. Case reports are professional narratives that provide feedback on clinical practice guidelines and offer a framework for early signals of effectiveness, adverse events, and cost. They can be shared for medical, scientific, or educational purposes.
In a randomized experiment, allocation concealment hides the sorting of trial participants into treatment groups so that this knowledge cannot be exploited. Adequate allocation concealment serves to prevent study participants from influencing treatment allocations for subjects. Studies with poor allocation concealment are prone to selection bias.
Consolidated Standards of Reporting Trials (CONSORT) encompasses various initiatives developed by the CONSORT Group to alleviate the problems arising from inadequate reporting of randomized controlled trials. It is part of the larger EQUATOR Network initiative to enhance the transparency and accuracy of reporting in research.
The STROBE(STrengthening the Reporting of OBservational studies in Epidemiology) Statement is a reporting guideline including a checklist of 22 items that are considered essential for good reporting of observational studies. It was published simultaneously in several leading biomedical journals in October and November 2007 and comprises both the checklist and an explanation and elaboration article which gives examples of good reporting and provides authors with more guidance on good reporting. It is also referred to in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals established by the International Committee of Medical Journal Editors and is endorsed by hundreds of biomedical journals.
Sir Rory Edwards Collins FMedSci FRS is a British physician who is Professor of Medicine and Epidemiology at the Clinical Trial Service Unit within the University of Oxford, the head of the Nuffield Department of Population Health and a Fellow of Green Templeton College, Oxford. His work has been in the establishment of large-scale epidemiological studies of the causes, prevention and treatment of heart attacks, other vascular disease, and cancer, while also being closely involved in developing approaches to the combination of results from related studies ("meta-analyses"). Since September 2005, he has been the Principal Investigator and Chief Executive of the UK Biobank, a prospective study of 500,000 British people aged 40–69 at recruitment.
Open peer review is the various possible modifications of the traditional scholarly peer review process. The three most common modifications to which the term is applied are:
- Open identities: Authors and reviewers are aware of each other's identity.
- Open reports: Review reports are published alongside the relevant article.
- Open participation: The wider community are able to contribute to the review process.

John P. A. Ioannidis is a Greek-American physician-scientist, writer and Stanford University professor who has made contributions to evidence-based medicine, epidemiology, and clinical research. Ioannidis studies scientific research itself, meta-research primarily in clinical medicine and the social sciences.
Critical appraisal in evidence based medicine, is the use of explicit, transparent methods to assess the data in published research, applying the rules of evidence to factors such as internal validity, adherence to reporting standards, conclusions, generalizability and risk-of-bias. Critical appraisal methods form a central part of the systematic review process. They are used in evidence synthesis to assist clinical decision-making, and are increasingly used in evidence-based social care and education provision.
A cluster-randomised controlled trial is a type of randomised controlled trial in which groups of subjects are randomised. Cluster randomised controlled trials are also known as cluster-randomised trials, group-randomised trials, and place-randomized trials. Cluster-randomised controlled trials are used when there is a strong reason for randomising treatment and control groups over randomising participants.
The Enhancing the Quality and Transparency of health research Network is an international initiative aimed at promoting transparent and accurate reporting of health research studies to enhance the value and reliability of medical research literature. The EQUATOR Network was established with the goals of raising awareness of the importance of good reporting of research, assisting in the development, dissemination and implementation of reporting guidelines for different types of study designs, monitoring the status of the quality of reporting of research studies in the health sciences literature, and conducting research relating to issues that impact the quality of reporting of health research studies. The Network acts as an "umbrella" organisation, bringing together developers of reporting guidelines, medical journal editors and peer reviewers, research funding bodies, and other key stakeholders with a mutual interest in improving the quality of research publications and research itself. The EQUATOR Network comprises four centres at the University of Oxford, Bond University, Paris Descartes University, and Ottawa Hospital Research Institute.

PRISMA is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard superseded the earlier QUOROM standard. It offers the replicability of a systematic literature review. Researchers have to figure out research objectives that answer the research question, states the keywords, a set of exclusion and inclusion criteria. In the review stage, relevant articles were searched, irrelevant ones are removed. Articles are analyzed according to some pre-defined categories.

Hugh MacPherson was a professor of acupuncture research at the University of York, founder and trustee of the Northern College of Acupuncture, founder and co-ordinator of the international STRICTA group, clinic director of York Clinic, fellow of The College of Medicine, and a practising member of the British Acupuncture Council.
PREDIMED was a large Spanish primary prevention trial which included 7,447 Spanish participants who were at high risk for cardiovascular disease, but otherwise healthy. They were randomly assigned to receive interventions with intensive education to one of three diets:
- Mediterranean diet supplemented with extra-virgin olive oil.
- Mediterranean diet supplemented with nuts.
- Control diet encouraging low-fat food items.
Allegiance bias in behavioral sciences is a bias resulted from the investigator's or researcher's allegiance to a specific school of thought. Researchers/investigators have been exposed to many types of branches of psychology or schools of thought. Naturally they adopt a school or branch that fits with their paradigm of thinking. More specifically, allegiance bias is when this leads therapists, researchers, etc. believing that their school of thought or treatment is superior to others. Their superior belief to these certain schools of thought can bias their research in effective treatments trials or investigative situations leading to allegiance bias. Reason being is that they may have devoted their thinking to certain treatments they have seen work in their past experiences. This can lead to errors in interpreting the results of their research. Their “pledge” to stay within their own paradigm of thinking may affect their ability to find more effective treatments to help the patient or situation they are investigating.
Peymané Adab is a British physician who is a Professor of Public Health at the University of Birmingham. She leads the Institute of Applied Health Research Chronic Disease Management Team. Adab investigates the epidemiology and management of obesity and chronic obstructive pulmonary disease.
A non-pharmaceutical intervention or non-pharmacological intervention (NPI) is any type of health intervention which is not primarily based on medication. Some examples include exercise, sleep improvement, or dietary habits.
References
- 1 2 Virginia Barbour publications indexed by Google Scholar
- 1 2 Virginia Barbour publications from Europe PubMed Central
- 1 2 3 4 5 Anon (2018). "Virginia Barbour: Queen of open access". BMJ. 363: k4148. doi:10.1136/bmj.k4148. ISSN 0959-8138. PMID 30355729. S2CID 53032870.
- ↑ Virginia Barbour on Twitter
- 1 2 Barbour, Virginia (1997). Regulation of the human α-globin genes by their chromatin context. jisc.ac.uk (DPhil thesis). University of Oxford. OCLC 43192909. EThOS uk.bl.ethos.244591.[ permanent dead link ]
- 1 2 3 "QUT | Staff Profiles | Ginny Barbour". staff.qut.edu.au. Retrieved 19 May 2019.
- 1 2 3 Tachibana, Chris (3 November 2017). "Responsibly conducting research". Science | AAAS. Retrieved 19 May 2019.
- 1 2 Couzin-Frankel, Jennifer (2018). "'Journalologists' use scientific methods to study academic publishing. Is their work improving science?". Science. doi:10.1126/science.aav4758. ISSN 0036-8075. S2CID 115360831.
- ↑ "Virginia Barbour | Committee on Publication Ethics: COPE". publicationethics.org. Retrieved 19 May 2019.
- ↑ Barbour, V M; Williams, P F (1990). "Nephrotic syndrome associated with sulphasalazine". BMJ. 301 (6755): 818. doi:10.1136/bmj.301.6755.818-b. ISSN 0959-8138. PMC 1663947 . PMID 1977483.
- ↑ Barbour, Virginia (2003). "UK Biobank: a project in search of a protocol?". The Lancet. 361 (9370): 1734–1738. doi:10.1016/S0140-6736(03)13377-6. ISSN 0140-6736. PMID 12767753. S2CID 37182739.
- ↑ Schulz, Kenneth F.; Altman, Douglas G.; Moher, David; the CONSORT Group (24 March 2010). "CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials". BMC Medicine. 8 (1): 18. doi: 10.1186/1741-7015-8-18 . ISSN 1741-7015. PMC 2860339 . PMID 20334633.
- ↑ Hoffmann, T. C.; Glasziou, P. P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D. G.; Barbour, V.; Macdonald, H.; Johnston, M.; Lamb, S. E.; Dixon-Woods, M.; McCulloch, P.; Wyatt, J. C.; Chan, A.-W.; Michie, S. (2014). "Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide". BMJ. 348 (mar07 3): g1687. doi: 10.1136/bmj.g1687 . ISSN 1756-1833. PMID 24609605.
- ↑ Shamseer, Larissa; Moher, David; Maduekwe, Onyi; Turner, Lucy; Barbour, Virginia; Burch, Rebecca; Clark, Jocalyn; Galipeau, James; Roberts, Jason; Shea, Beverley J. (2017). "Potential predatory and legitimate biomedical journals: can you tell the difference? A cross-sectional comparison". BMC Medicine. 15 (1): 28. doi: 10.1186/s12916-017-0785-9 . ISSN 1741-7015. PMC 5353955 . PMID 28298236.