1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s.
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.

1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula C
2H
4Br
2. Although trace amounts occur naturally in the ocean, where it is probably formed by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint, sweet odor, detectable at 10 ppm, and is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive.

Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. The basic structure is shared by parathion methyl.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2.

Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's Silent Spring questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades. In the United States, the Environmental Protection Agency has limited the sale of heptachlor products to the specific application of fire ant control in underground transformers. The amount that can be present in different foods is regulated.

Captan is a general use pesticide (GUP) that belongs to the phthalimide class of fungicides. It is a white solid, although commercial samples appear yellow or brownish.
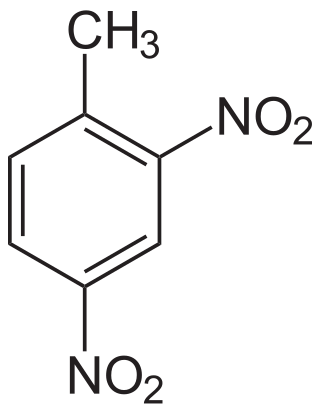
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.

Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized impurities. Diphenylamine dissolves well in many common organic solvents, and is moderately soluble in water. It is used mainly for its antioxidant properties. Diphenylamine is widely used as an industrial antioxidant, dye mordant and reagent and is also employed in agriculture as a fungicide and antihelmintic.
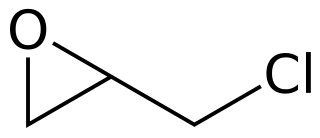
Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.

Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses. The compound is a slightly viscous liquid that is slightly unstable and is not often encountered in pure form.

Vinyl bromide is a simple vinyl halide. It is a colorless liquid. It is produced from ethylene dibromide. It is mainly used as a comonomer to confer fire retardant properties to acrylate polymers.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.

Hexachlorobutadiene, (often abbreviated as "HCBD") Cl2C=C(Cl)C(Cl)=CCl2, is a colorless liquid at room temperature that has an odor similar to that of turpentine. It is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds. Structurally, it has a 1,3-butadiene core, but fully substituted with chlorine atoms.

1,2-Dichloropropane is an organic compound classified as a chlorocarbon. It is a colorless, flammable liquid with a sweet odor. it is obtained as a byproduct of the production of epichlorohydrin, which is produced on a large scale.
4,4′-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless solid, although commercial samples can appear yellow or brown. It is produced on an industrial scale, mainly as a precursor to polyurethanes.

1,2,3-Trichloropropane (TCP) is an organic compound with the formula CHCl(CH2Cl)2. It is a colorless liquid that is used as a solvent and in other specialty applications.