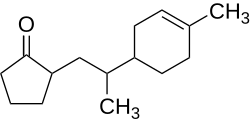
Properties
The flash point of the compound is 162.5 °C, and the autoignition temperature is 294 °C. [1] The specific rotation is reported to be [α]D20=+228–235° (1 M; chloroform) [2]
In general, the compound features a fruity apricot-like odor. Of the four stereo isomers, (2R,2′S,1′′R)-Nectaryl and (2R,2′R,1′′R)-Nectaryl contribute especially to the compound's odor, the odor detection threshold lies at 0.094 ng·l−1 and 0.112 ng·l−1, respectively. In contrast to that, the other stereo isomers show an unspecific fruity odor, the odor detection threshold are 11.2 ng·l−1 and 14.9 ng·l−1 which is much higher. [2] [3]
The tenacity on blotter, the time during which the compound is smellable with unchanged characteristics, [4] : 51 is reported to be three weeks. [4] : 52

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Rose oil is the essential oil extracted from the petals of various types of rose. Rose ottos are extracted through steam distillation, while rose absolutes are obtained through solvent extraction, the absolute being used more commonly in perfumery. The production technique originated in Greater Iran. Even with their high price and the advent of organic synthesis, rose oils are still perhaps the most widely used essential oil in perfumery.

Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.
The odor detection threshold is the lowest concentration of a certain odor compound that is perceivable by the human sense of smell. The threshold of a chemical compound is determined in part by its shape, polarity, partial charges, and molecular mass. The olfactory mechanisms responsible for a compound's different detection threshold is not well understood. As such, odor thresholds cannot be accurately predicted. Rather, they must be measured through extensive tests using human subjects in laboratory settings.

Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.

Methyl dihydrojasmonate is an aroma compound that smells similar to jasmine. In racemic mixtures the odor is floral and citrus while epimerized mixtures exhibit a dense buttery-floral odor with odor recognition thresholds of 15 parts per billion.

Philip Kraft is a German organic chemist. Since 1996 he has been employed by Givaudan, a leading Flavor and Fragrance company, where he designs captive odorants for use in perfumes. He has lectured at the University of Bern, the University of Zurich, and the ETH Zurich.
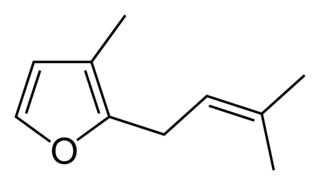
Rosefuran (3-methyl-2-prenylfuran) is an organic compound, classified as a terpenoid. It is a minor constituent of the aroma of the rose. Rosefuran is a 2,3-disubstituted furan. It has an odor threshold of 200 ppb and constitutes 0.16% of Bulgarian rose oil. Rosefuran has been established as a female sex pheromone of an acarid mite, Caloglyphus sp. Concentrations of less than 100 ng of synthetic rosefuran caused sexual excitation in males of the species.

Pomarose is a high-impact captive odorant patented by Givaudan. It is a double-unsaturated ketone that does not occur in nature. Pomarose has a powerful fruity rose odor with nuances of apples, plums and raisins, which is almost entirely due to the (2E,5Z)-stereoisomer, while its (2E,5E)-isomer is barely detectable for most people. Catalyzed by traces of acids, both isomers equilibrate however quickly upon standing in glass containers.
Decenoic acid is any mono-carboxylic acid with an unbranched chain of ten carbons connected by eight single bonds and one double bond; that is, a chemical compound with formula HO(O=)C–(CH
2)
k–CH=CH–(CH
2)
7-k–H, where k is between 0 and 7 inclusive.

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.
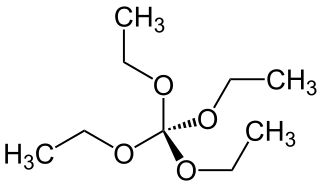
Tetraethoxymethane is a chemical compound which is formally formed by complete ethylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarbonic acid violates the Erlenmeyer rule and is unstable in free state).
2-Methylbutanoic acid, also known as 2-methylbutyric acid is a branched-chain alkyl carboxylic acid with the chemical formula CH3CH2CH(CH3)CO2H, classified as a short-chain fatty acid. It exists in two enantiomeric forms, (R)- and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic occurs in many fruits such as apples and apricots, as well as in the scent of the orchid Luisia curtisii.
Pentenoic acid is any of five mono-carboxylic acids whose molecule has an unbranched chain of five carbons connected by three single bonds and one double bond. That is, any compound with one of the formulas HO(O=)C−CH=CH−CH2−CH3 (2-pentenoic), HO(O=)C−CH2−CH=CH−CH3 (3-pentenoic), or HO(O=)C−CH2−CH2−CH=CH2 (4-pentenoic). In the IUPAC-recommended nomenclature, these acids are called pent-2-enoic, pent-3-enoic, and pent-4-enoic, respectively. All these compounds have the empirical formula C
5H
8O
2.