
A flavoring, also known as flavor or flavorant, is a food additive used to improve the taste or smell of food. It changes the perceptual impression of food as determined primarily by the chemoreceptors of the gustatory and olfactory systems. Along with additives, other components like sugars determine the taste of food.

This article talks about TCA, aka Tricloroanisole.

Methanethiol is an organosulfur compound with the chemical formula CH
3SH. It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals, as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.
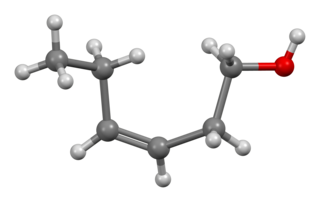
cis-3-Hexen-1-ol, also known as (Z)-3-hexen-1-ol and leaf alcohol, is a colorless oily liquid with an intense grassy-green odor of freshly cut green grass and leaves. It is produced in small amounts by most plants and it acts as an attractant to many predatory insects. cis-3-Hexen-1-ol is a very important aroma compound that is used in fruit and vegetable flavors and in perfumes. The yearly production is about 30 tonnes.

Petrichor is the earthy scent produced when rain falls on dry soil. The word is constructed from Ancient Greek πέτρα (pétra) 'rock', or πέτρος (pétros) 'stone', and ἰχώρ (ikhṓr), the ethereal fluid that is the blood of the gods in Greek mythology.
The vibration theory of smell proposes that a molecule's smell character is due to its vibrational frequency in the infrared range. This controversial theory is an alternative to the more widely accepted docking theory of olfaction, which proposes that a molecule's smell character is due to a range of weak non-covalent interactions between its protein odorant receptor, such as electrostatic and Van der Waals interactions as well as H-bonding, dipole attraction, pi-stacking, metal ion, Cation–pi interaction, and hydrophobic effects, in addition to the molecule's conformation.
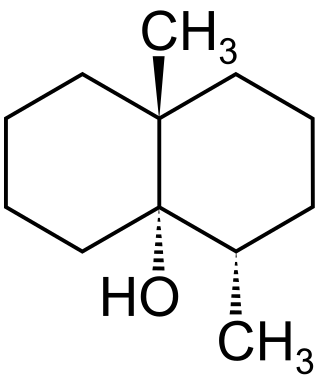
Geosmin ( jee-OZ-min) is an irregular sesquiterpenoid, produced from the universal sesquiterpene precursor farnesyl pyrophosphate (also known as farnesyl diphosphate), in a two-step Mg2+-dependent reaction. Geosmin, along with the irregular monoterpene 2-methylisoborneol, together account for the majority of biologically-caused taste and odor outbreaks in drinking water worldwide. Geosmin has a distinct earthy or musty odor, which most people can easily smell. The geosmin odor detection threshold in humans is very low, ranging from 0.006 to 0.01 micrograms per liter in water. Geosmin is also responsible for the earthy taste of beetroots and a contributor to the strong scent (petrichor) that occurs in the air when rain falls after a spell of dry weather or when soil is disturbed.
A wine fault is a sensory-associated (organoleptic) characteristic of a wine that is unpleasant, and may include elements of taste, smell, or appearance, elements that may arise from a "chemical or a microbial origin", where particular sensory experiences might arise from more than one wine fault. Wine faults may result from poor winemaking practices or storage conditions that lead to wine spoilage.
Grapefruit mercaptan is a natural organic compound found in grapefruit. It is a monoterpenoid that contains a thiol functional group. Structurally a hydroxy group of terpineol is replaced by the thiol in grapefruit mercaptan, so it also called thioterpineol. Volatile thiols typically have very strong, often unpleasant odors that can be detected by humans in very low concentrations. Grapefruit mercaptan has a very potent, but not unpleasant, odor, and it is the chemical constituent primarily responsible for the aroma of grapefruit. This characteristic aroma is a property of only the R enantiomer.
Olfactory fatigue, also known as odor fatigue, olfactory adaptation, and noseblindness, is the temporary, normal inability to distinguish a particular odor after a prolonged exposure to that airborne compound. For example, when entering a restaurant initially the odor of food is often perceived as being very strong, but after time the awareness of the odor normally fades to the point where the smell is not perceptible or is much weaker. After leaving the area of high odor, the sensitivity is restored with time. Anosmia is the permanent loss of the sense of smell, and is different from olfactory fatigue.

An electronic nose is an electronic sensing device intended to detect odors or flavors. The expression "electronic sensing" refers to the capability of reproducing human senses using sensor arrays and pattern recognition systems.
Alkylpyrazines are chemical compounds based on pyrazine with different substitution patterns. Some alkylpyrazines are naturally occurring highly aromatic substances which often have a very low odor threshold and contribute to the taste and aroma of various foods including cocoa, baked goods, coffee and wines. Alkylpyrazines are also formed during the cooking of some foods via Maillard reactions.

Food pairing is a method of identifying which foods go well together from a flavor standpoint, often based on individual tastes, popularity, availability of ingredients, and traditional cultural practices.

An odor or odour is caused by one or more volatilized chemical compounds that are generally found in low concentrations that humans and many animals can perceive via their sense of smell. An odor is also called a "smell" or a "scent", which can refer to either an unpleasant or a pleasant odor.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
A key odorant is a volatile compound that is present in concentrations higher than their specific flavor threshold.
The olfactory system is the system related to the sense of smell (olfaction). Many fish activities are dependent on olfaction, such as: mating, discriminating kin, avoiding predators, locating food, contaminant avoidance, imprinting and homing. These activities are referred to as “olfactory-mediated.” Impairment of the olfactory system threatens survival and has been used as an ecologically relevant sub-lethal toxicological endpoint for fish within studies. Olfactory information is received by sensory neurons, like the olfactory nerve, that are in a covered cavity separated from the aquatic environment by mucus. Since they are in almost direct contact with the surrounding environment, these neurons are vulnerable to environmental changes. Fish can detect natural chemical cues in aquatic environments at concentrations as low as parts per billion (ppb) or parts per trillion (ppt).

Insect olfaction refers to the function of chemical receptors that enable insects to detect and identify volatile compounds for foraging, predator avoidance, finding mating partners and locating oviposition habitats. Thus, it is the most important sensation for insects. Most important insect behaviors must be timed perfectly which is dependent on what they smell and when they smell it. For example, olfaction is essential for locating host plants and hunting prey in many species of insects, such as the moth Deilephila elpenor and the wasp Polybia sericea, respectively.
Gas chromatography-olfactometry (GC-O) is a technique that integrates the separation of volatile compounds using a gas chromatograph with the detection of odour using an olfactometer. It was first invented and applied in 1964 by Fuller and co-workers. While GC separates volatile compounds from an extract, human olfaction detects the odour activity of each eluting compound. In this olfactometric detection, a human assessor may qualitatively determine whether a compound has odour activity or describe the odour perceived, or quantitatively evaluate the intensity of the odour or the duration of the odour activity. The olfactometric detection of compounds allows the assessment of the relationship between a quantified substance and the human perception of its odour, without instrumental detection limits present in other kinds of detectors. Compound identification still requires use of other detectors, such as mass spectrometry, with analytical standards.











