
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Isoquinoline is an individual chemical specimen - a heterocyclic aromatic organic compound - as well as the name of a family of many thousands of natural plant alkaloids, any one of which might be referred to as "an isoquinoline". It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in many naturally occurring alkaloids such as papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
Thiazole, or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

2,6-Lutidine is a natural heterocyclic aromatic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivative of pyridine, all of which are referred to as lutidines. It is a colorless liquid with mildly basic properties and a pungent, noxious odor.
Picoline refers to any of three isomers of methylpyridine (CH3C5H4N). They are all colorless liquids with a characteristic smell similar to that of pyridine. They are miscible with water and most organic solvents.

Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions. It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.
2-Methylpyridine, or 2-picoline, is the compound described with formula C6H7N. 2-Picoline is a colorless liquid that has an unpleasant odor similar to pyridine. It is mainly used to make vinylpyridine and the agrichemical nitrapyrin.
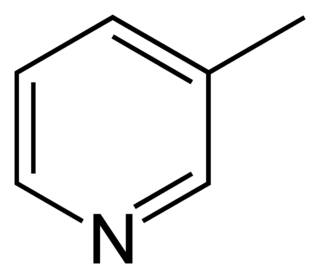
3-Methylpyridine or 3-picoline, is an organic compound with formula 3-CH3C5H4N. It is one of three positional isomers of methylpyridine, whose structures vary according to where the methyl group is attached around the pyridine ring. This colorless liquid is a precursor to pyridine derivatives that have applications in the pharmaceutical and agricultural industries. Like pyridine, 3-methylpyridine is a colorless liquid with a strong odor and is classified as a weak base.

2-Mercaptopyridine is an organosulfur compound with the formula HSC5H4N. This yellow crystalline solid is a derivative of pyridine. The compound and its derivatives serve primarily as acylating agents. A few of 2-mercaptopyridine’s other uses include serving as a protecting group for amines and imides as well as forming a selective reducing agent. 2-Mercaptopyridine oxidizes to 2,2’-dipyridyl disulfide.
Chloropyridines are a group of aryl chlorides consisting of a pyridine ring with chlorine atoms as substituents.

Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidines.

Pyrithione is the common name of an organosulfur compound with molecular formula C
5H
5NOS, chosen as an abbreviation of pyridinethione, and found in the Persian shallot. It exists as a pair of tautomers, the major form being the thione 1-hydroxy-2(1H)-pyridinethione and the minor form being the thiol 2-mercaptopyridine N-oxide; it crystallises in the thione form. It is usually prepared from either 2-bromopyridine, 2-chloropyridine, or 2-chloropyridine N-oxide, and is commercially available as both the neutral compound and its sodium salt. It is used to prepare zinc pyrithione, which is used primarily to treat dandruff and seborrhoeic dermatitis in medicated shampoos, though is also an anti-fouling agent in paints.
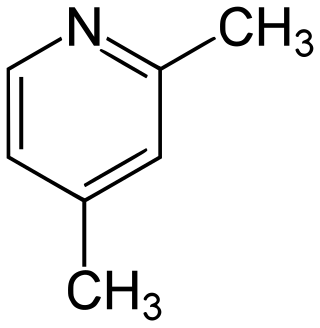
2,4-Lutidine is a heterocyclic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivatives of pyridine, all of which are referred to as lutidines. It is a colorless liquid with mildly basic properties and a pungent, noxious odor. The compound has few uses.
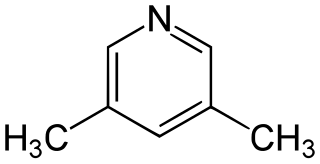
3,5-Lutidine is a heterocyclic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivatives of pyridine, all of which are referred to as lutidines. It is a colorless liquid with mildly basic properties and a pungent odor. The compound is a precursor to the drug omeprazole.
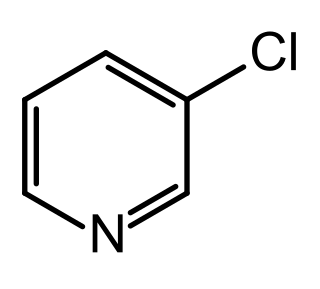
3-Chloropyridine is an aryl chloride and isomer of chloropyridine with the formula C5H4ClN. It is a colorless liquid that is mainly used as a building block in organic synthesis.























