ADAMs are a family of single-pass transmembrane and secreted metalloendopeptidases. All ADAMs are characterized by a particular domain organization featuring a pro-domain, a metalloprotease, a disintegrin, a cysteine-rich, an epidermal-growth factor like and a transmembrane domain, as well as a C-terminal cytoplasmic tail. Nonetheless, not all human ADAMs have a functional protease domain, which indicates that their biological function mainly depends on protein–protein interactions. Those ADAMs which are active proteases are classified as sheddases because they cut off or shed extracellular portions of transmembrane proteins. For example, ADAM10 can cut off part of the HER2 receptor, thereby activating it. ADAM genes are found in animals, choanoflagellates, fungi and some groups of green algae. Most green algae and all land plants likely lost ADAM proteins.
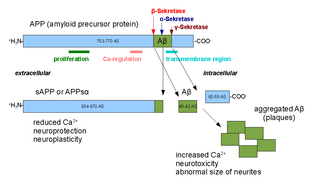
Alpha secretases are a family of proteolytic enzymes that cleave amyloid precursor protein (APP) in its transmembrane region. Specifically, alpha secretases cleave within the fragment that gives rise to the Alzheimer's disease-associated peptide amyloid beta when APP is instead processed by beta secretase and gamma secretase. The alpha-secretase pathway is the predominant APP processing pathway. Thus, alpha-secretase cleavage precludes amyloid beta formation and is considered to be part of the non-amyloidogenic pathway in APP processing. Alpha secretases are members of the ADAM family, which are expressed on the surfaces of cells and anchored in the cell membrane. Several such proteins, notably ADAM10, have been identified as possessing alpha-secretase activity. Upon cleavage by alpha secretases, APP releases its extracellular domain - a fragment known as APPsα - into the extracellular environment in a process known as ectodomain shedding.
The nuclear receptor coactivator 2 also known as NCoA-2 is a protein that in humans is encoded by the NCOA2 gene. NCoA-2 is also frequently called glucocorticoid receptor-interacting protein 1 (GRIP1), steroid receptor coactivator-2 (SRC-2), or transcriptional mediators/intermediary factor 2 (TIF2).

Phosphatidylinositol 3-kinase regulatory subunit alpha is an enzyme that in humans is encoded by the PIK3R1 gene.

Insulin-like growth factor-binding protein 3, also known as IGFBP-3, is a protein that in humans is encoded by the IGFBP3 gene. IGFBP-3 is one of six IGF binding proteins that have highly conserved structures and bind the insulin-like growth factors IGF-1 and IGF-2 with high affinity. IGFBP-7, sometimes included in this family, shares neither the conserved structural features nor the high IGF affinity. Instead, IGFBP-7 binds IGF1R, which blocks IGF-1 and IGF-2 binding, resulting in apoptosis.

Disintegrin and metalloproteinase domain-containing protein 15 is an enzyme that in humans is encoded by the ADAM15 gene.
Alpha-actinin-2 is a protein which in humans is encoded by the ACTN2 gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.
A disintegrin and metalloproteinase with thrombospondin motifs 1 is an enzyme that in humans is encoded by the ADAMTS1 gene.

ARF GTPase-activating protein GIT1 is an enzyme that in humans is encoded by the GIT1 gene.

Endophilin-A1 is a protein that in humans is encoded by the SH3GL2 gene.

Disintegrin and metalloproteinase domain-containing protein 9 is an enzyme that in humans is encoded by the ADAM9 gene.

Laminin subunit beta-2 is a protein that in humans is encoded by the LAMB2 gene.
Tetranectin is a protein that in humans is encoded by the CLEC3B gene.

Sorting nexin-9 is a protein that in humans is encoded by the SNX9 gene.

ADAM19 , is a human gene.

Integrin alpha-9 is a protein that in humans is encoded by the ITGA9 gene. Cytogenetic location: 3p22.2

Protein kinase C and casein kinase substrate in neurons protein 3 is an enzyme that in humans is encoded by the PACSIN3 gene.

Disintegrin and metalloproteinase domain-containing protein 28 is an enzyme that in humans is encoded by the ADAM28 gene.
A disintegrin and metalloprotease 3, or ADAM3, belongs to a family of peptidase proteins referred to as ADAMs. Many of these are solely found in spermatogenic cells, specifically in the anterior portion of capacitated spermatozoa heads. This membrane protein is critical for crucial steps in fertilization such as migration of sperm through the uterus to the oviduct as well as binding to the zona pellucida. Inactivation of ADAM3 is a cause of male infertility.