
Yersinia pestis is a gram-negative, non-motile, coccobacillus bacterium without spores that is related to both Yersinia pseudotuberculosis and Yersinia enterocolitica. It is a facultative anaerobic organism that can infect humans via the Oriental rat flea. It causes the disease plague, which caused the first plague pandemic and the Black Death, the deadliest pandemic in recorded history. Plague takes three main forms: pneumonic, septicemic, and bubonic. Yersinia pestis is a parasite of its host, the rat flea, which is also a parasite of rats, hence Y. pestis is a hyperparasite.

Hydroxylysine (Hyl) is an amino acid with the molecular formula C6H14N2O3. It was first discovered in 1921 by Donald Van Slyke as the 5-hydroxylysine form. It arises from a post-translational hydroxy modification of lysine. It is most widely known as a component of collagen.
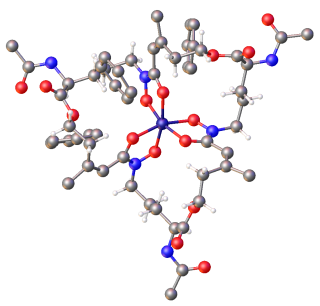
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated. Siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:

The gcvB RNA gene encodes a small non-coding RNA involved in the regulation of a number of amino acid transport systems as well as amino acid biosynthetic genes. The GcvB gene is found in enteric bacteria such as Escherichia coli. GcvB regulates genes by acting as an antisense binding partner of the mRNAs for each regulated gene. This binding is dependent on binding to a protein called Hfq. Transcription of the GcvB RNA is activated by the adjacent GcvA gene and repressed by the GcvR gene. A deletion of GcvB RNA from Y. pestis changed colony shape as well as reducing growth. It has been shown by gene deletion that GcvB is a regulator of acid resistance in E. coli. GcvB enhances the ability of the bacterium to survive low pH by upregulating the levels of the alternate sigma factor RpoS. A polymeric form of GcvB has recently been identified. Interaction of GcvB with small RNA SroC triggers the degradation of GcvB by RNase E, lifting the GcvB-mediated mRNA repression of its target genes.

RyhB RNA is a 90 nucleotide RNA that down-regulates a set of iron-storage and iron-using proteins when iron is limiting; it is itself negatively regulated by the ferric uptake repressor protein, Fur.

In enzymology, a L-lysine 6-monooxygenase (NADPH) (EC 1.14.13.59) is an enzyme that catalyzes the chemical reaction
In enzymology, an aerobactin synthase (EC 6.3.2.39) is an enzyme that catalyzes the chemical reaction
In enzymology, a N6-hydroxylysine O-acetyltransferase (EC 2.3.1.102) is an enzyme that catalyzes the chemical reaction

In molecular biology, LcrV is a protein found in Yersinia pestis and several other bacterial species. It forms part of the Yersinia pestis virulence protein factors that also includes all Yops, or Yersinia outer protein, but the name has been kept out of convention. LcrV's main function is not actually known, but it is essential for the production of other Yops.

The IscR stability element is a conserved secondary structure found in the intergenic regions of iscRSUA polycistronic mRNA. This secondary structure prevents the degradation of the iscR mRNA.

Yersiniabactin (Ybt) is a siderophore found in the pathogenic bacteria Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica, as well as several strains of enterobacteria including enteropathogenic Escherichia coli and Salmonella enterica. Siderophores, compounds of low molecular mass with high affinities for ferric iron, are important virulence factors in pathogenic bacteria. Iron—an essential element for life used for such cellular processes as respiration and DNA replication—is extensively chelated by host proteins like lactoferrin and ferritin; thus, the pathogen produces molecules with an even higher affinity for Fe3+ than these proteins in order to acquire sufficient iron for growth. As a part of such an iron-uptake system, yersiniabactin plays an important role in pathogenicity of Y. pestis, Y. pseudotuberculosis, and Y. entercolitica.

Escherichia coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but pathogenic varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans. Unlike normal flora E. coli, the pathogenic varieties produce toxins and other virulence factors that enable them to reside in parts of the body normally not inhabited by E. coli, and to damage host cells. These pathogenic traits are encoded by virulence genes carried only by the pathogens.

In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis,Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.
Ferric-chelate reductase (NADPH) (EC 1.16.1.9, ferric chelate reductase, iron chelate reductase, NADPH:Fe3+-EDTA reductase, NADPH-dependent ferric reductase, yqjH (gene)) is an enzyme with systematic name Fe(II):NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction

OmpT is an aspartyl protease found on the outer membrane of Escherichia coli. OmpT is a subtype of the family of omptin proteases, which are found on some gram-negative species of bacteria.
The alpha-D-phosphohexomutases are a large superfamily of enzymes, with members in all three domains of life. Enzymes from this superfamily are ubiquitous in organisms from E. Coli to humans, and catalyze a phosphoryl transfer reaction on a phosphosugar substrate. Four well studied subgroups in the superfamily are:
- Phosphoglucomutase (PGM)
- Phosphoglucomutase/Phosphomannomutase (PGM/PMM)
- Phosphoglucosamine mutase (PNGM)
- Phosphoaceytlglucosamine mutase (PAGM)
N2-citryl-N6-acetyl-N6-hydroxylysine synthase (EC 6.3.2.38, N(alpha)-citryl-N(epsilon)-acetyl-N(epsilon)-hydroxylysine synthase, iucA (gene)) is an enzyme with systematic name citrate:N6-acetyl-N6-hydroxy-L-lysine ligase (ADP-forming). This enzyme catalyses the following chemical reaction
P fimbriae or P pili or Pap are chaperon-usher type fimbrial appendages found on the surface of many Escherichia coli bacteria. The P fimbriae is considered to be one of the most important virulence factor in uropathogenic E. coli and plays an important role in upper urinary tract infections. P fimbriae mediate adherence to host cells, a key event in the pathogenesis of urinary tract infections.

Virginia L. Miller is a microbiologist known for her work on studying the factors leading to disease caused by bacteria. Miller is an elected fellow of the American Academy of Microbiology (2003) and a former Pew Charitable Trust Biomedical Scholar (1989).













