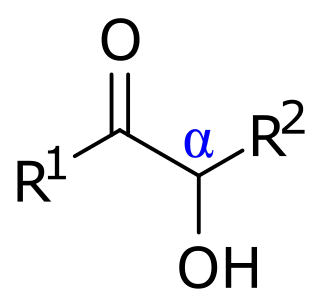In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. Simply, this is the structural unit of carbohydrates.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are nonsuperposable onto their own mirror image. Enantiomers are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
In stereochemistry, diastereomers are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is superposable on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.

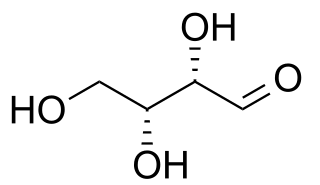
Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides. The threose name can be used to refer to both the D- and L-stereoisomers, and more generally to the racemic mixture (D/L-, equal parts D- and L-) as well as to the more generic threose structure (absolute stereochemistry unspecified).

Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (galactaric acid or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.
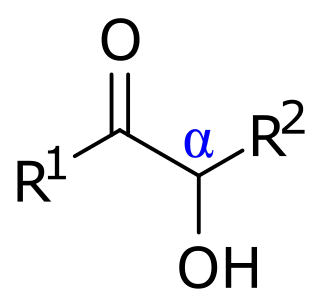
In organic chemistry, acyloins or α-hydroxy ketones are a class of organic compounds of the general form R−C(=O)−CR'(OH)−R", composed of a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. They are one of the two main classes of hydroxy ketones, distinguished by the position of the hydroxy group relative to the ketone; in this form, the hydroxy is on the alpha carbon, explaining the secondary name of α-hydroxy ketone.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
The Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Emil Fischer, is a method for synthesizing monosaccharides. It proceeds via synthesis and hydrolysis of a cyanohydrin, followed by reduction of the intermediate acid to the aldehyde, thus elongating the carbon chain of an aldose by one carbon atom while preserving stereochemistry on all the previously present chiral carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomeric sugars, called epimers. For example, D-arabinose is converted to a mixture of D-glucose and D-mannose.

Uronic acids or alduronic acids are a class of sugar acids with both carbonyl and carboxylic acid functional groups. They are sugars in which the hydroxyl group furthest from the carbonyl group has been oxidized to a carboxylic acid. Usually the sugar is an aldose, but fructuronic acid also occurs. Oxidation of the terminal aldehyde instead yields an aldonic acid, while oxidation of both the terminal hydroxyl group and the aldehyde yields an aldaric acid. The names of uronic acids are generally based on their parent sugars, for example, the uronic acid analog of glucose is glucuronic acid. Uronic acids derived from hexoses are known as hexuronic acids and uronic acids derived from pentoses are known as penturonic acids.

An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. Thus, their general chemical formula is HO2C(CHOH)nCH2OH. Oxidation of the terminal hydroxyl group instead of the terminal aldehyde yields a uronic acid, while oxidation of both terminal ends yields an aldaric acid.

Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules where carbon is bonded to four different substituents. This type of construction creates two possible enantiomers. Absolute configuration uses a set of rules to describe the relative positions of each bond around the chiral center atom. The most common labeling method uses the descriptors R or S and is based on the Cahn–Ingold–Prelog priority rules. R and S refer to rectus and sinister, Latin for right and left, respectively.