In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
In polymer chemistry, emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomers, and surfactants. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
Sizing or size is a substance that is applied to, or incorporated into, other materials—especially papers and textiles—to act as a protective filler or glaze. Sizing is used in papermaking and textile manufacturing to change the absorption and wear characteristics of those materials.

In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and as a frame material for eyeglasses; it is also used as a synthetic fiber in the manufacture of cigarette filters and playing cards. In photographic film, cellulose acetate film replaced nitrate film in the 1950s, being far less flammable and cheaper to produce.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.

Straight-chain terminal alkenes, also called linear alpha olefins (LAO) or normal alpha olefins (NAO), are alkenes (olefins) having a chemical formula CnH2n, distinguished from other alkenes with a similar molecular formula by being terminal alkenes, in which the double bond occurs at the alpha position, and by having a linear (unbranched) hydrocarbon chain.
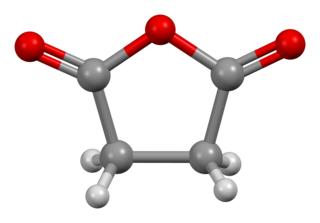
Succinic anhydride, is an organic compound with the molecular formula (CH2CO)2O. This colorless solid is the acid anhydride of succinic acid.

Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is a white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis.
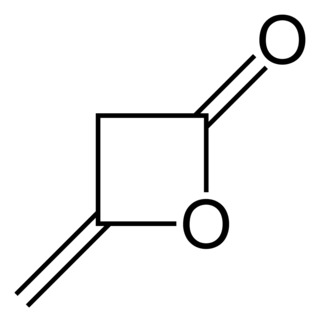
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.
The wet strength of paper and paperboard is a measure of how well the web of fibers holding the paper together can resist a force of rupture when the paper is wet. Wet strength is routinely expressed as the ratio of wet to dry tensile force at break.
Microcrystalline cellulose (MCC) is a term for refined wood pulp and is used as a texturizer, an anti-caking agent, a fat substitute, an emulsifier, an extender, and a bulking agent in food production. The most common form is used in vitamin supplements or tablets. It is also used in plaque assays for counting viruses, as an alternative to carboxymethylcellulose.
In polymer chemistry, cationic polymerization is a type of chain growth polymerization in which a cationic initiator transfers charge to a monomer, which then becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer. The types of monomers necessary for cationic polymerization are limited to alkenes with electron-donating substituents and heterocycles. Similar to anionic polymerization reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain. Cationic polymerization is used in the production of polyisobutylene and poly(N-vinylcarbazole) (PVK).

Paper chemicals designate a group of chemicals that are used for paper manufacturing, or modify the properties of paper. These chemicals can be used to alter the paper in many ways, including changing its color and brightness, or by increasing its strength and resistance to water. The chemicals can be defined on basis of their usage in the process.
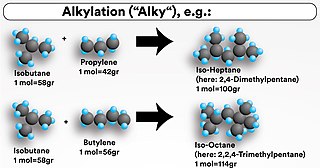
An alkylation unit (alky) is one of the conversion processes used in petroleum refineries. It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline component. The process occurs in the presence of an acid such as sulfuric acid (H2SO4) or hydrofluoric acid (HF) as catalyst. Depending on the acid used, the unit is called a sulfuric acid alkylation unit (SAAU) or hydrofluoric acid alkylation unit (HFAU). In short, the alky produces a high-quality gasoline blending stock by combining two shorter hydrocarbon molecules into one longer chain gasoline-range molecule by mixing isobutane with a light olefin such as propylene or butylene from the refinery's fluid catalytic cracking unit (FCCU) in the presence of an acid catalyst.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.
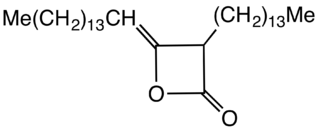
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.
Vinylene carbonate (VC) or 1,3-dioxol-2-one, is the simplest unsaturated cyclic carbonic acid ester. Vinylene carbonate can also be thought of as the cyclic carbonate of the hypothetical (Z)-ethene-1,2-diol. The activated double bond in this five-membered oxygen-containing heterocycle makes the molecule a reactive monomer for homopolymerization and copolymerization and a dienophile in Diels-Alder reactions. Below room temperature vinylene carbonate is a colorless stable solid.
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.