In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
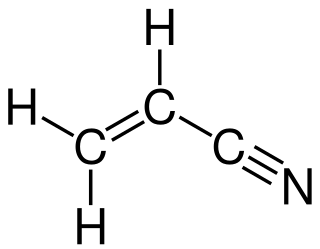
Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
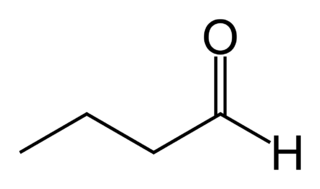
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.
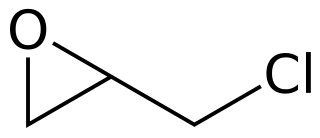
Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.

Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

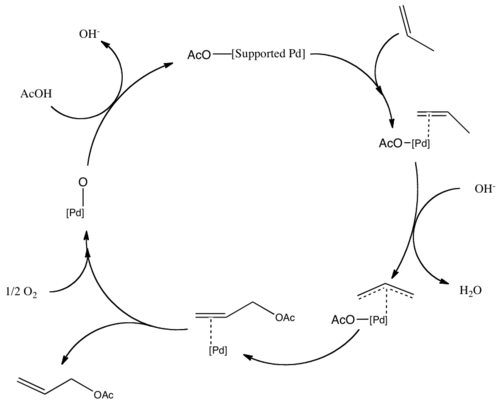
Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:

Allyl cyanide is an organic compound with the formula CH2CHCH2CN. Like other small alkyl nitriles, allyl cyanide is colorless and soluble in organic solvents. Allyl cyanide occurs naturally as an antifeedant and is used as a cross-linking agent in some polymers.

1-Octanol, also known as octan-1-ol, is the organic compound with the molecular formula CH3(CH2)7OH. It is a fatty alcohol. Many other isomers are also known generically as octanols. 1-Octanol is manufactured for the synthesis of esters for use in perfumes and flavorings. It has a pungent odor. Esters of octanol, such as octyl acetate, occur as components of essential oils. It is used to evaluate the lipophilicity of pharmaceutical products.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.