Adolph Wilhelm Hermann Kolbe was a major contributor to the birth of modern organic chemistry. He was a professor at Marburg and Leipzig. Kolbe was the first to apply the term synthesis in a chemical context, and contributed to the philosophical demise of vitalism through synthesis of the organic substance acetic acid from carbon disulfide, and also contributed to the development of structural theory. This was done via modifications to the idea of "radicals" and accurate prediction of the existence of secondary and tertiary alcohols, and to the emerging array of organic reactions through his Kolbe electrolysis of carboxylate salts, the Kolbe-Schmitt reaction in the preparation of aspirin and the Kolbe nitrile synthesis. After studies with Wöhler and Bunsen, Kolbe was involved with the early internationalization of chemistry through work in London. He was elected to the Royal Swedish Academy of Sciences, and won the Royal Society of London's Davy Medal in the year of his death. Despite these accomplishments and his training important members of the next generation of chemists, Kolbe is best remembered for editing the Journal für Praktische Chemie for more than a decade, in which his vituperative essays on Kekulé's structure of benzene, van't Hoff's theory on the origin of chirality and Baeyer's reforms of nomenclature were personally critical and linguistically violent. Kolbe died of a heart attack in Leipzig at age 68, six years after the death of his wife, Charlotte. He was survived by four children.
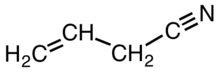
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals. The reaction produces an acyloin. In the classic application benzaldehyde is converted to benzoin.
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.

Carl Dietrich Harries was a German chemist born in Luckenwalde, Brandenburg, Prussia. He received his doctorate in 1892. In 1900, he married Hertha von Siemens, daughter of the electrical genius Werner von Siemens, and the inventor of one of the earliest ozone generators. In 1904, he moved as full professor to the University of Kiel, where he remained until 1916. During that time he published numerous papers on ozonolysis. His major publication detailing ozonolysis was published in Liebigs Ann. Chem. 1905, 343, 311. Dissatisfied with academic life and having failed to obtain either of two positions at universities, he left academia to become Director of Research at Siemens and Halske. He died on 3 November 1923 of complications following surgery for cancer.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.

Wilhelm Rudolph Fittig was a German chemist. Fittig discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. He studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine naphthalene and fluorene.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed. The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.

Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser.

Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.

Jakob Meisenheimer was a German chemist. He made numerous contributions to organic chemistry, the most famous being his proposed structure for a group of compounds now named Meisenheimer complex. He also proposed the mechanism of the Beckmann rearrangement. Later in his career, he reported the synthesis of the pyridine-N-oxide.

The Rosenmund–von Braun synthesis is an organic reaction in which an aryl halide reacts with cuprous cyanide to yield an aryl nitrile.
The Dimroth rearrangement is a rearrangement reaction taking place with certain 1,2,3-triazoles where endocyclic and exocyclic nitrogen atoms switch place. This organic reaction was discovered in 1909 by Otto Dimroth.

Piprozolin is a medication for bile therapy.
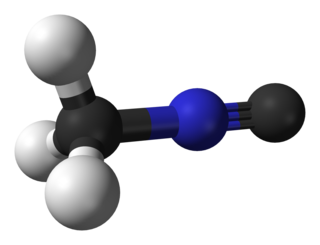
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric to methyl cyanide (acetonitrile), but its reactivity is very different. In contrast to the faintly sweet, ethereal odor of acetonitrile, the smell of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.
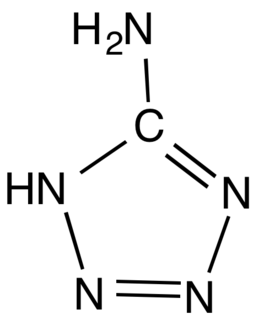
5-Aminotetrazole is an organic compound with the formula HN4CNH2. It is a white solid that can be obtained both in anhydrous and hydrated forms.