Brassicaceae or Cruciferae is a medium-sized and economically important family of flowering plants commonly known as the mustards, the crucifers, or the cabbage family. Most are herbaceous plants, while some are shrubs. The leaves are simple, lack stipules, and appear alternately on stems or in rosettes. The inflorescences are terminal and lack bracts. The flowers have four free sepals, four free alternating petals, two shorter free stamens and four longer free stamens. The fruit has seeds in rows, divided by a thin wall.

Horseradish is a perennial plant of the family Brassicaceae. It is a root vegetable, cultivated and used worldwide as a spice and as a condiment. The species is probably native to Southeastern Europe and Western Asia.

Broccoli is an edible green plant in the cabbage family whose large flowering head, stalk and small associated leaves are eaten as a vegetable. Broccoli is classified in the Italica cultivar group of the species Brassica oleracea. Broccoli has large flower heads, or florets, usually dark green, arranged in a tree-like structure branching out from a thick stalk which is usually light green. The mass of flower heads is surrounded by leaves. Broccoli resembles cauliflower, which is a different but closely related cultivar group of the same Brassica species.

Brassica is a genus of plants in the cabbage and mustard family (Brassicaceae). The members of the genus are informally known as cruciferous vegetables, cabbages, mustard plants, or simply brassicas. Crops from this genus are sometimes called cole crops—derived from the Latin caulis, denoting the stem or stalk of a plant.

In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.

Mustard oil can mean either the pressed oil used for cooking, or a pungent essential oil also known as volatile oil of mustard. The essential oil results from grinding mustard seed, mixing the grounds with water, and isolating the resulting volatile oil by distillation. It can also be produced by dry distillation of the seed. Pressed mustard oil is used as cooking oil in some cultures, but sale is restricted in some countries due to high levels of erucic acid. Varieties of mustard seed also exist that are low in erucic acid.

Allyl isothiocyanate (AITC) is a naturally occurring unsaturated isothiocyanate. The colorless oil is responsible for the pungent taste of Cruciferous vegetables such as mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.
Nitrilase enzymes catalyse the hydrolysis of nitriles to carboxylic acids and ammonia, without the formation of "free" amide intermediates. Nitrilases are involved in natural product biosynthesis and post translational modifications in plants, animals, fungi and certain prokaryotes. Nitrilases can also be used as catalysts in preparative organic chemistry. Among others, nitrilases have been used for the resolution of racemic mixtures. Nitrilase should not be confused with nitrile hydratase which hydrolyses nitriles to amides. Nitrile hydratases are almost invariably co-expressed with an amidase, which converts the amide to the carboxylic acid. Consequently, it can sometimes be difficult to distinguish nitrilase activity from nitrile hydratase plus amidase activity.

Sulforaphane is a compound within the isothiocyanate group of organosulfur compounds. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant, which allows the two compounds to mix and react.

Glucoraphanin is a glucosinolate found in broccoli, mustard and other cruciferous vegetables.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.
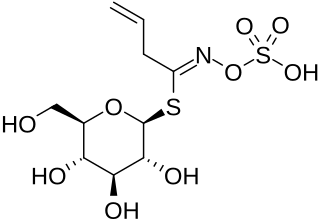
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard. Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil, which is responsible for the pungent taste of mustard and horseradish. Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.

Cruciferous vegetables are vegetables of the family Brassicaceae with many genera, species, and cultivars being raised for food production such as cauliflower, cabbage, kale, garden cress, bok choy, broccoli, Brussels sprouts, mustard plant and similar green leaf vegetables. The family takes its alternative name from the shape of their flowers, whose four petals resemble a cross.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.

Rhamphospermum arvense, the charlock mustard, field mustard, wild mustard, or just charlock, is an annual or winter annual plant in the family Brassicaceae. It is found in the fields of North Africa, Asia, Europe, and some other areas where it has been transported and naturalized. Pieris rapae, the small white butterfly, and Pieris napi, the green veined white butterfly, are significant consumers of charlock during their larval stages.
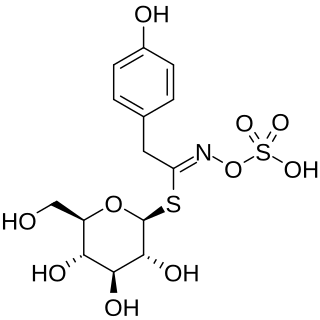
Sinalbin is a glucosinolate found in the seeds of white mustard, Sinapis alba, and in many wild plant species. In contrast to mustard from black mustard seeds which contain sinigrin, mustard from white mustard seeds has only a weakly pungent taste.

Gluconasturtiin or phenethyl glucosinolate is one of the most widely distributed glucosinolates in the cruciferous vegetables, mainly in the roots, and is probably one of the plant compounds responsible for the natural pest-inhibiting properties of growing crucifers, such as cabbage, mustard or rape, in rotation with other crops. This effect of gluconasturtiin is due to its degradation by the plant enzyme myrosinase into phenethyl isothiocyanate, which is toxic to many organisms.

Brevicoryne brassicae, commonly known as the cabbage aphid or cabbage aphis, is a destructive aphid native to Europe that is now found in many other areas of the world. The aphids feed on many varieties of produce, including cabbage, broccoli (especially), Brussels sprouts, cauliflower and many other members of the genus Brassica, but do not feed on plants outside of the family Brassicaceae. The insects entirely avoid plants other than those of Brassicaceae; even though thousands may be eating broccoli near strawberries, the strawberries will be left untouched.
The mustard oil bomb, formerly known as the glucosinolate–myrosinase complex, is a chemical herbivory defense system found in members of the Brassicaceae. The mustard oil bomb requires the activation of a common plant secondary metabolite, glucosinolate, by an enzyme, myrosinase. The defense complex is typical among plant defenses to herbivory in that the two molecules are stored in different compartments in the leaves of plants until the leaf is torn by an herbivore. The glucosinolate has a β-glucose and a sulfated oxime. The myrosinase removes the β-glucose to form mustard oils that are toxic to herbivores. The defense system was named a "bomb" by Matile, because it like a real bomb is waiting to detonate upon disturbance of the plant tissue.

Glucotropaeolin or benzyl glucosinolate is a glucosinolate found in cruciferous vegetables, particularly garden cress. Upon enzymatic activity, it is transformed into benzyl isothiocyanate, which contributes to the characteristic flavor of these brassicas.